Reinforced bioreactor component structure system for cell cultivation
a bioreactor and component structure technology, applied in the field of cell culture cell culture bags, can solve the problems of requiring a large space, unable to handle multiple patient samples at the same time, and cell culture was stand alone, so as to prevent crimping of the tube structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
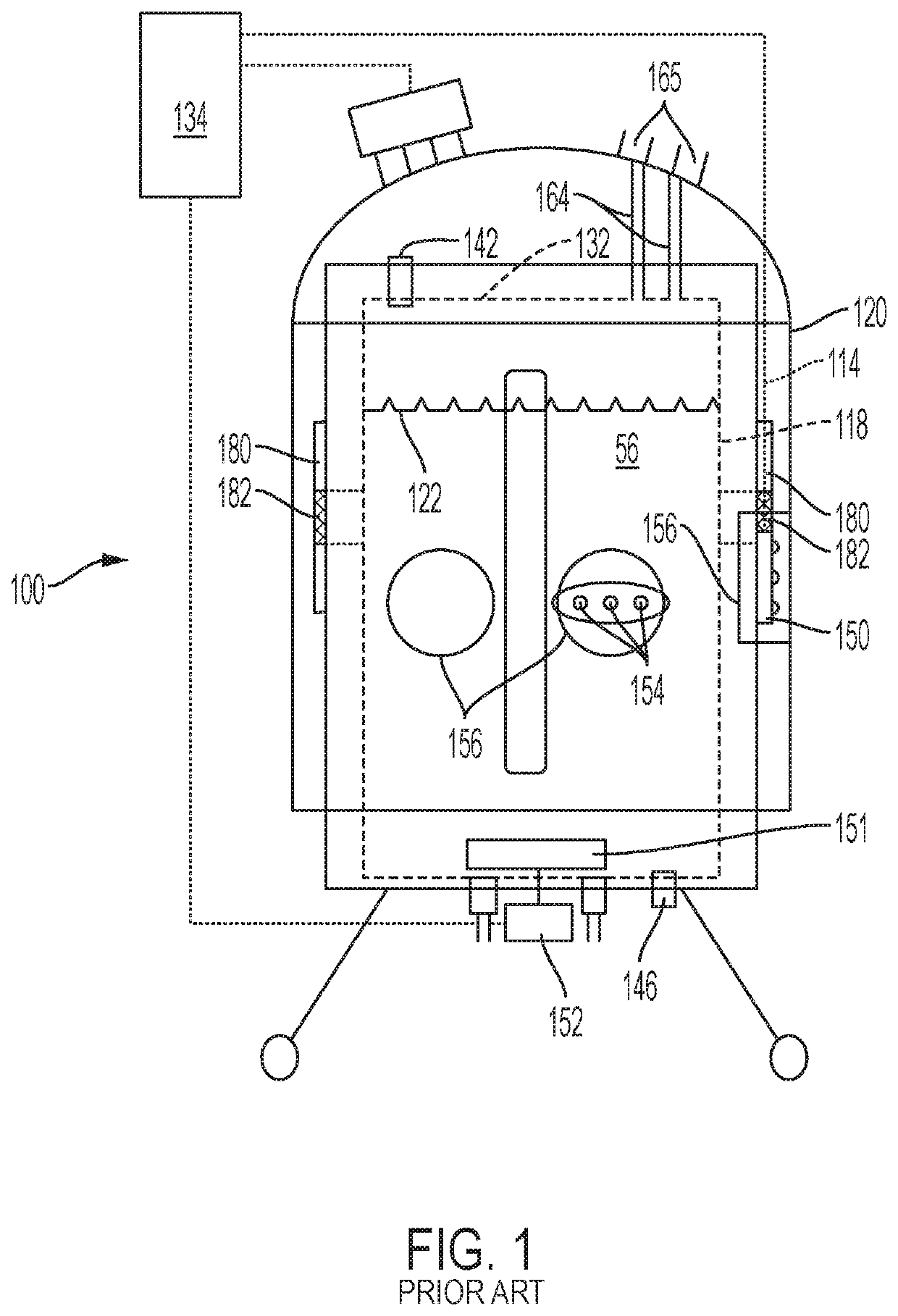
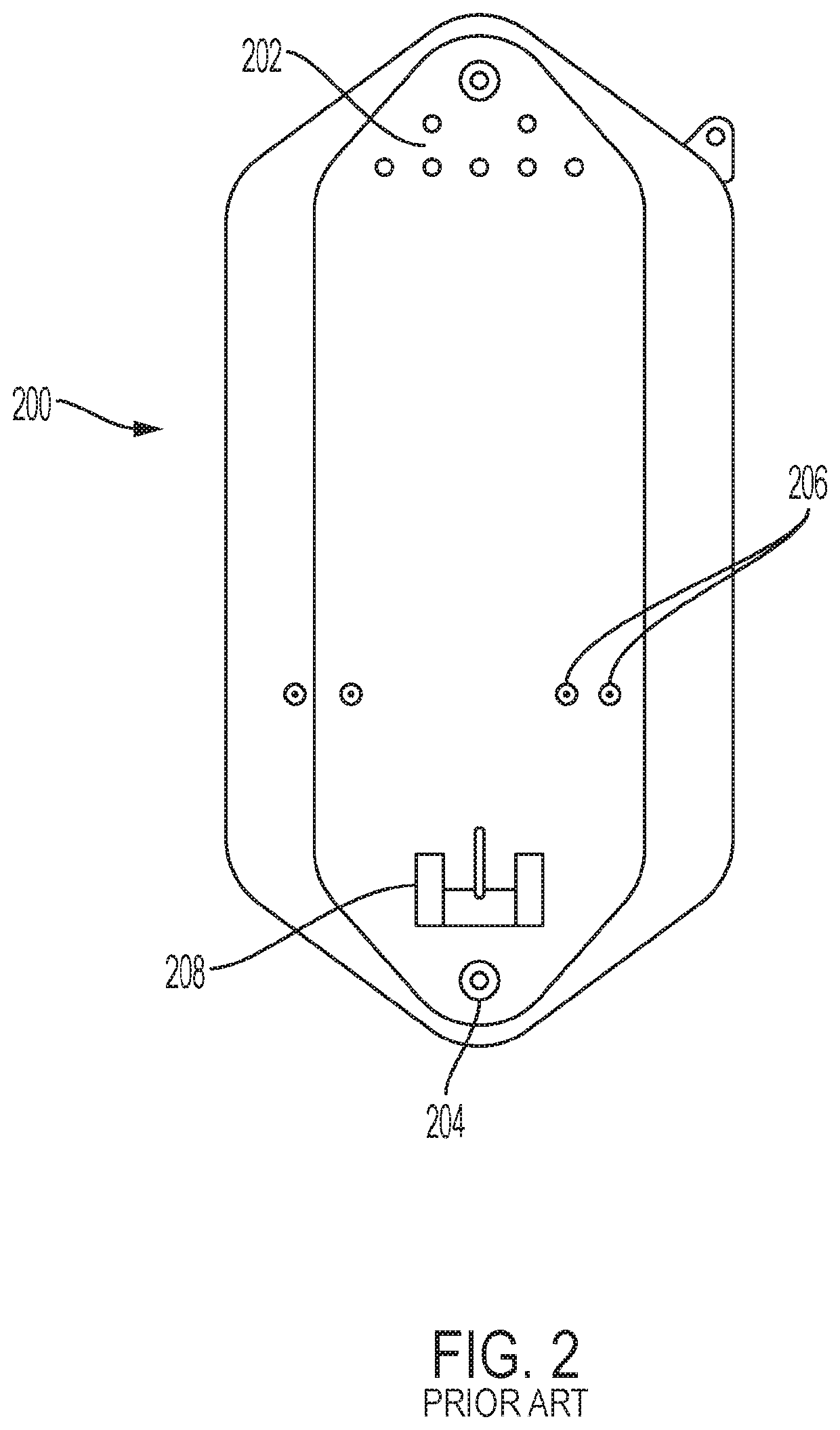
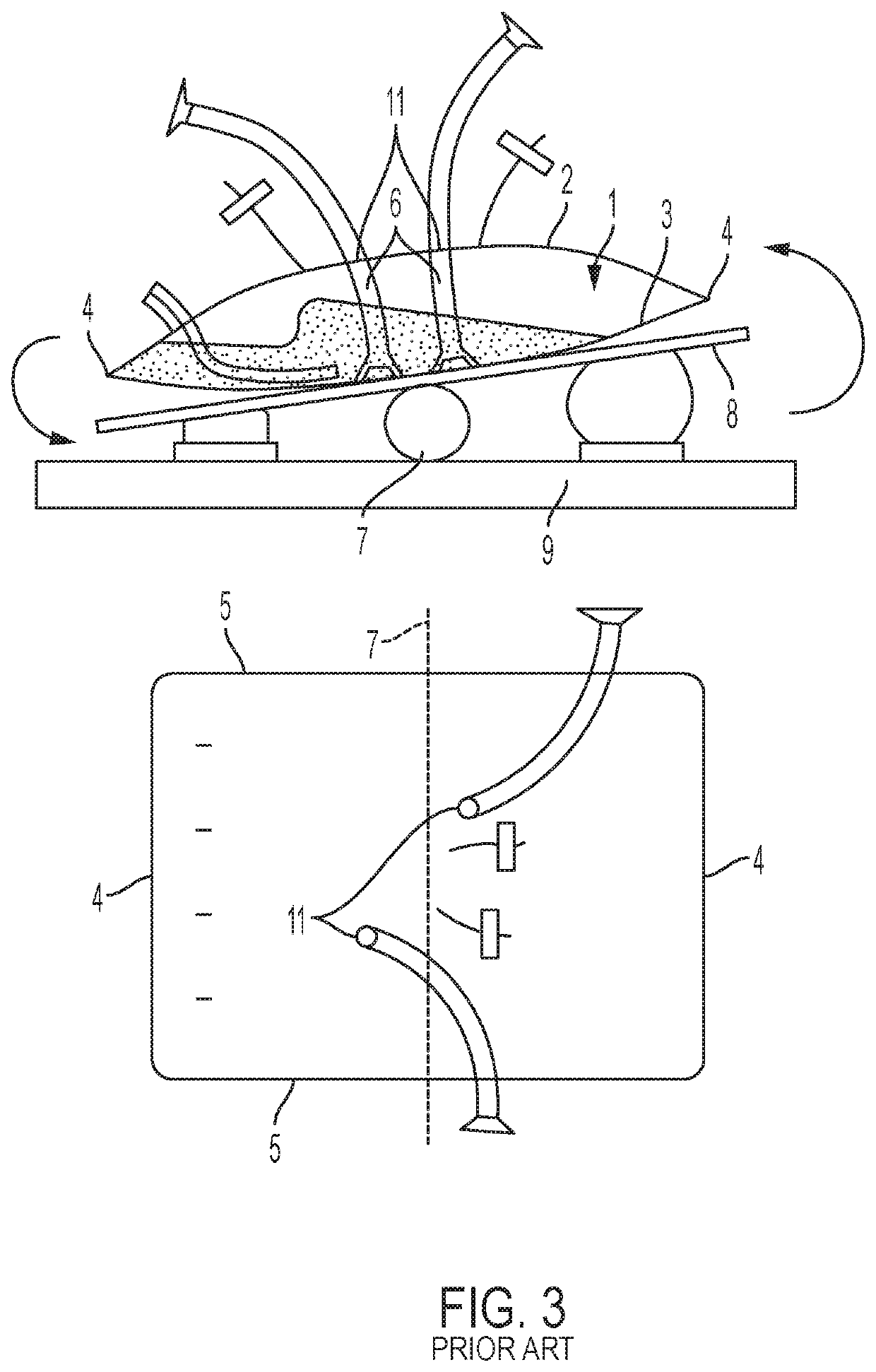
[0027]The present inventors have found that several of the internal structures of a bioreactor or fermenter bag are susceptible to damage or perceived damage as a result of sterilization and packaging, and further damage to these elements can occur during shipping, setup, or use. The sterilization of the bag with gamma irradiation can in some cases exacerbate the problem by fusing adjacent pieces of material or freezing the material into bent shape. With respect to parts that are internal to the bag, these problems are sometimes compounded by the inability to inspect internal portions of the bag given the need to maintain sterility and not compromise the bag structure. The perception of damage to an internal part may result in the entire bag being deemed unsuitable for use. The tube structure maybe adapted to accept a probe, such as a thermocouple, or a sparging wand. Alternatively, or in addition, the tube structure may be adapted for introducing or withdrawing material from the bi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com