Adoptive cell transfer and oncolytic virus combination therapy
a technology of oncolytic virus and cell transfer, which is applied in the field of conjugated immunotherapy methods for cancer treatment, can solve the problems of increasing healthcare costs, reducing the quality of life of patients, and affecting the survival rate of patients, and achieves the effect of increasing the selectivity of the virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
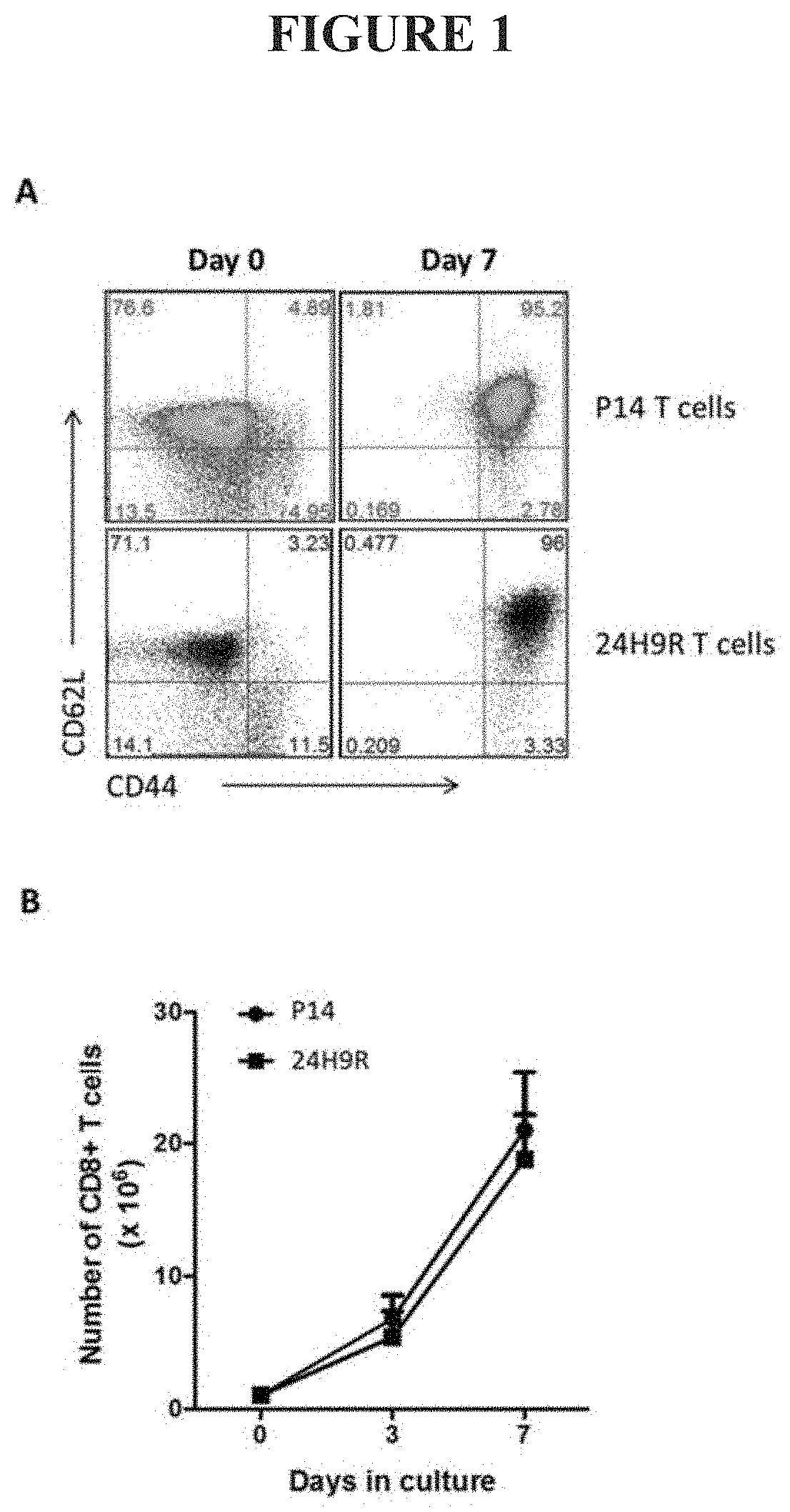
Ex Vivo Culture of TAA-Specific CD8 T Cells Induces a Central Memory Phenotype
[0085]Ex vivo based T cell cultures commonly utilize cytokines that signal through the common gamma chain (IL2Rγ or CD132). Common gamma chain family cytokines include, but are not limited to IL2, IL15, and IL21 [27]. CD132 is mainly expressed on lymphocytes and signaling through this receptor drives proliferation and maturation of CD8+ T cells and other lymphocyte subtypes [27]. IL2 is known to induce rapid proliferation and effector differentiation of CD8 T cells [28-30] whereas IL15 and IL21 are known to drive slower, homeostatic proliferation and bias CD8+ T cells towards a central memory differentiation status [30-32]. Therefore, we utilized IL15, and IL21 supplementation in our ex vivo culture system. In addition, we also employed rapamycin, a commonly used mTOR inhibitor. Regulation of the mTOR pathway has been shown to support survival of T cells and induce memory T cell differentiation in viral va...
example 2
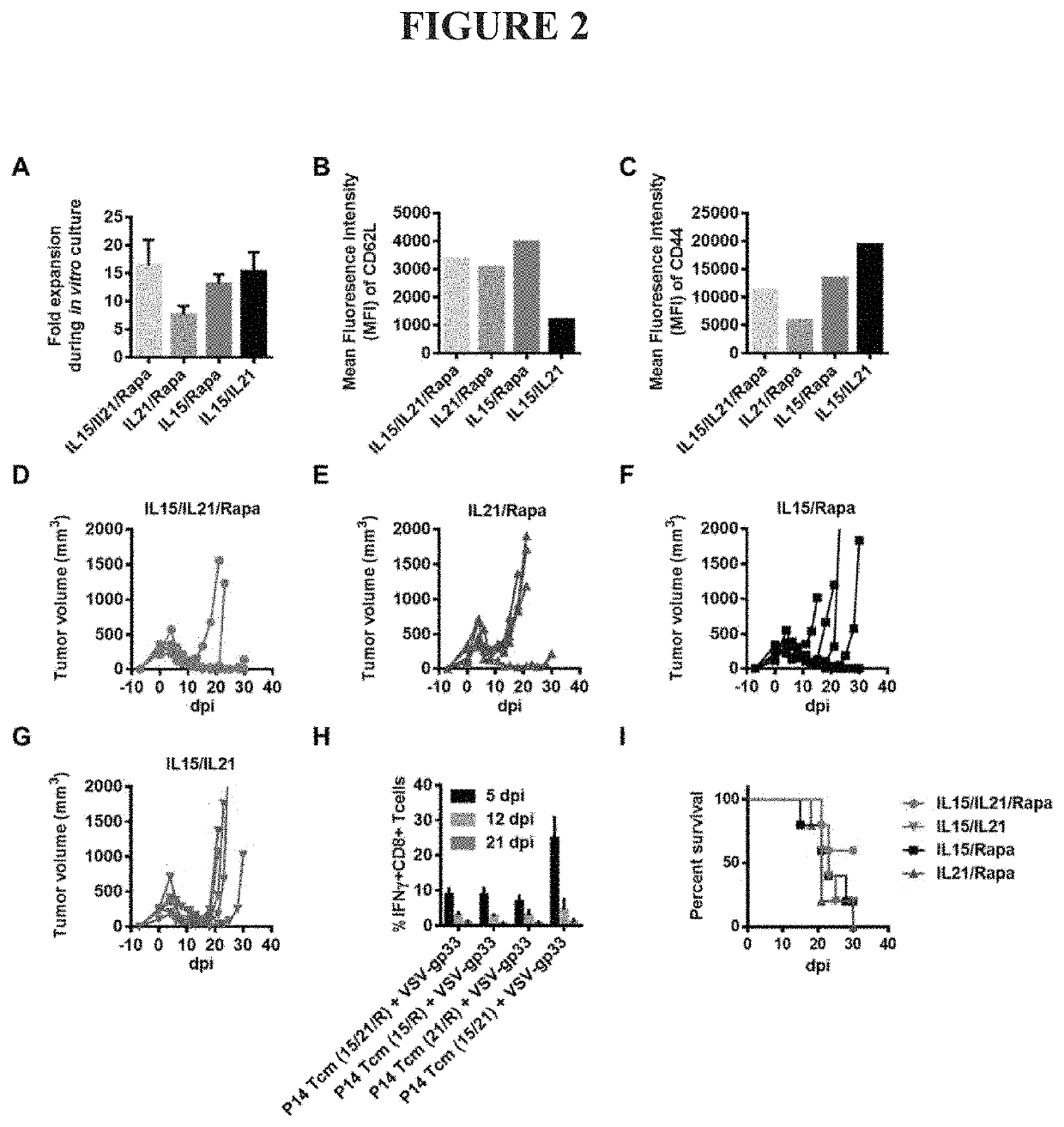
[0087]The Specific Combination of IL21, IL15, and Rapamycin is Required for Guided Differentiation of Antigen-Specific Central Memory CD8+ T Cells with Optimal Anti-Tumor Effect in the Combination Therapy.
[0088]The specific combination of rapamycin, IL15, and IL21 is required for ex vivo culture, expansion and differentiation of antigen-specific central memory CD8+ T cells with optimal anti-tumor effect in the combination therapy described herein. Each, and all, of these components are required to generate such Tcm cells and cells produced in the absence of one or more of these components have sub-optimal T cell expansion and / or reduced anti-tumor effect after in vivo infusion and OV vaccination.
[0089]IL15 is required in the culture protocol to drive CD8+ T cell expansion and central memory differentiation. Cells cultured in the absence of IL15 showed impaired proliferation and a reduced cell yield from the ex vivo culture (IL21 / Rapa in FIG. 2A) compared to cells grown in the full c...
example 3
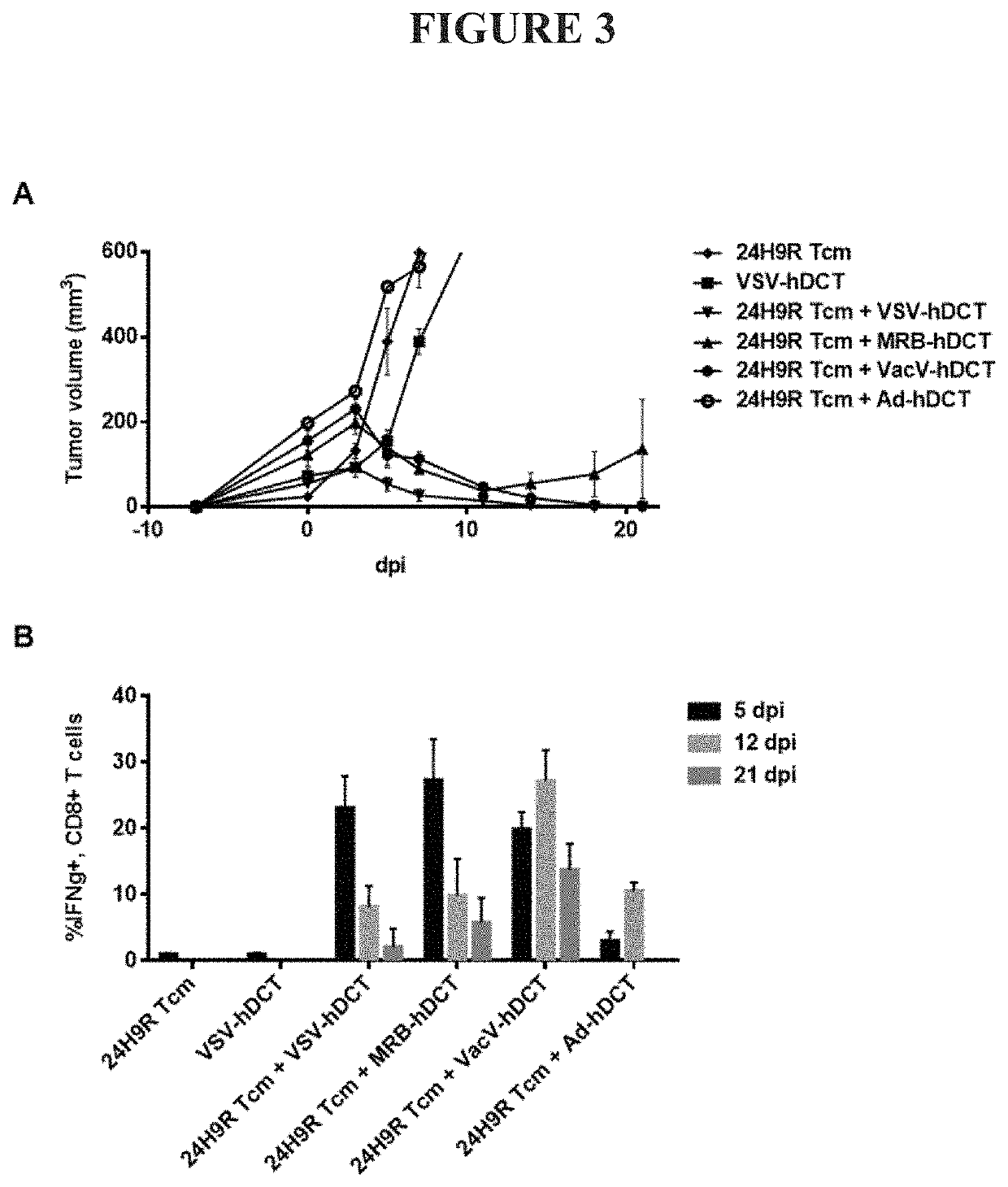
Transfer of Ex Vivo Cultured Memory T Cells Requires OV Vaccine Boost to Acquire Anti-Tumor Activity
[0092]Central memory CD8 T cells reside in the lymph nodes and remain inactive until stimulated via presentation with their cognate peptide. Upon antigen-specific stimulation, Tcm cells rapidly divide and differentiate into effector cells to generate a robust systemic cytolytic response. The antigen-specific cytolytic function of ex vivo culture generated Tcm cells was tested via adoptive transfer into mice bearing tumors expressing the antigen targeted by the transferred cells followed by activation with an oncolytic virus vaccine expressing the same antigen. We tested three TCR transgenic mouse strains (24H9R, DUC18, and P14 mice) which target antigens expressed on commonly used tumor cell line models representing a true self antigen, a neoantigen and a viral antigen (DCT in B16F10 cells, Erk9M in DUC18 cells, and B16-gp33 cells, respectively). Cultured Tcm cells were adoptively tra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com