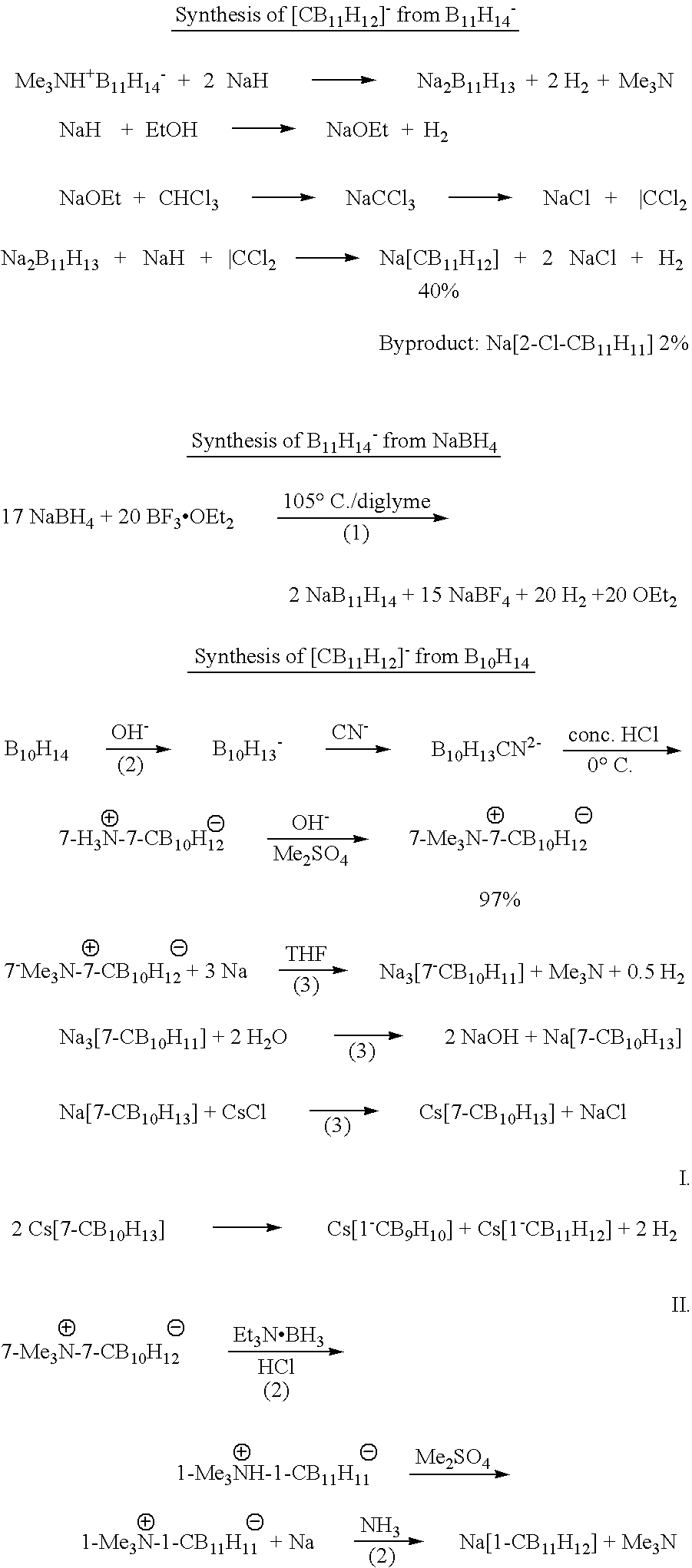Method for preparation of carborane anions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020]Synthesis of [Me3NH]+[CB11H12]−: from [Me3NH]+[B11H14]−; CCl2 from CHCl3 / NaH+EtOH, medium scale. In a 2 L two-neck flask [Me3NH]+[B11H14]−[1] (20 g, 0.104 mol) was dissolved in THF (200 mL) under an argon atmosphere. The solution was cooled to 0° C. and NaH (95%) (23 g, 1 mol) was added carefully. After stirring 30 min at room temperature, the THF and NMe3 were removed in vacuum and THF (400 mL) and CHCl3 (30 ml, 0.375 mol) were added. The reaction mixture was stirred for 2 h at ambient temperature. EtOH (80 ml) were added dropwise at 0° C. and it was stirred for 4 h at room temperature. Water (600 mL) was added and the THF was removed in vacuum and the solution was acidified by addition of 10% HCl. Residual THF and EtOH were removed in vacuum. [Me3NH]+Cl−(20 g, 0.2 mol) was added, and a white solid precipitated, which was dried in vacuum to yield a colorless mixture of [Me3NH]+[CB11H12]−(8.9 g, 42%) containing 2% of a [Me3NH]+[2-Cl-1-CB11H12]− contaminant as judged by 11B NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com