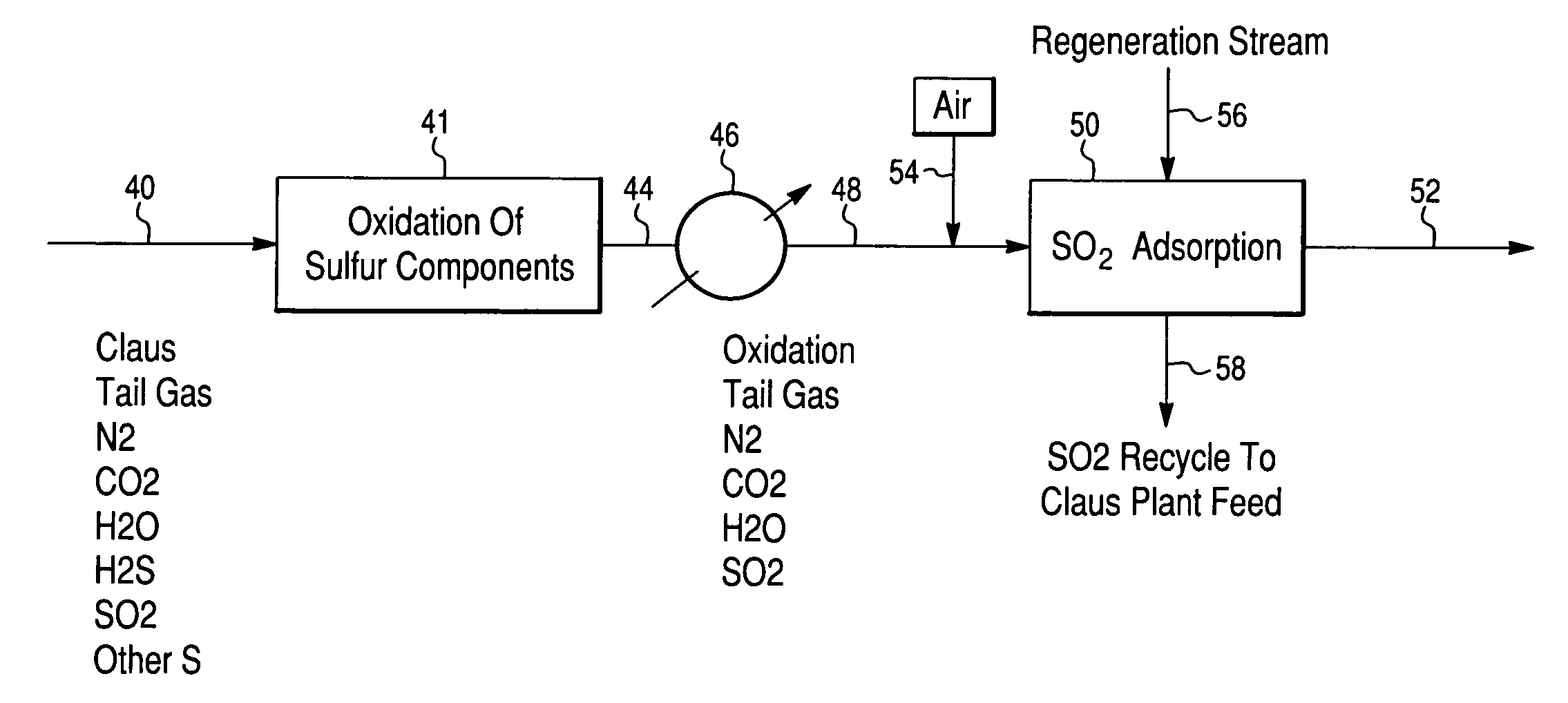
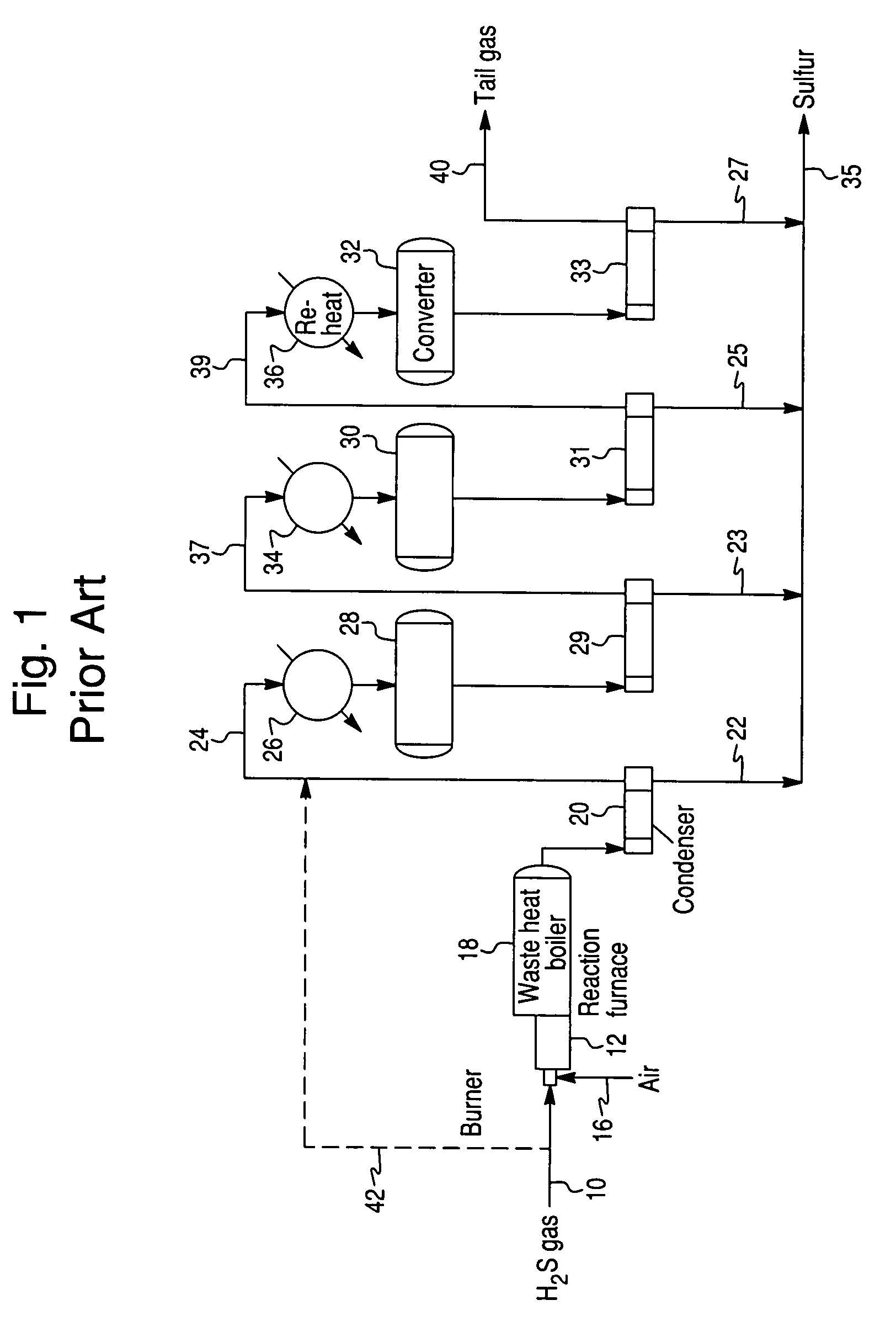
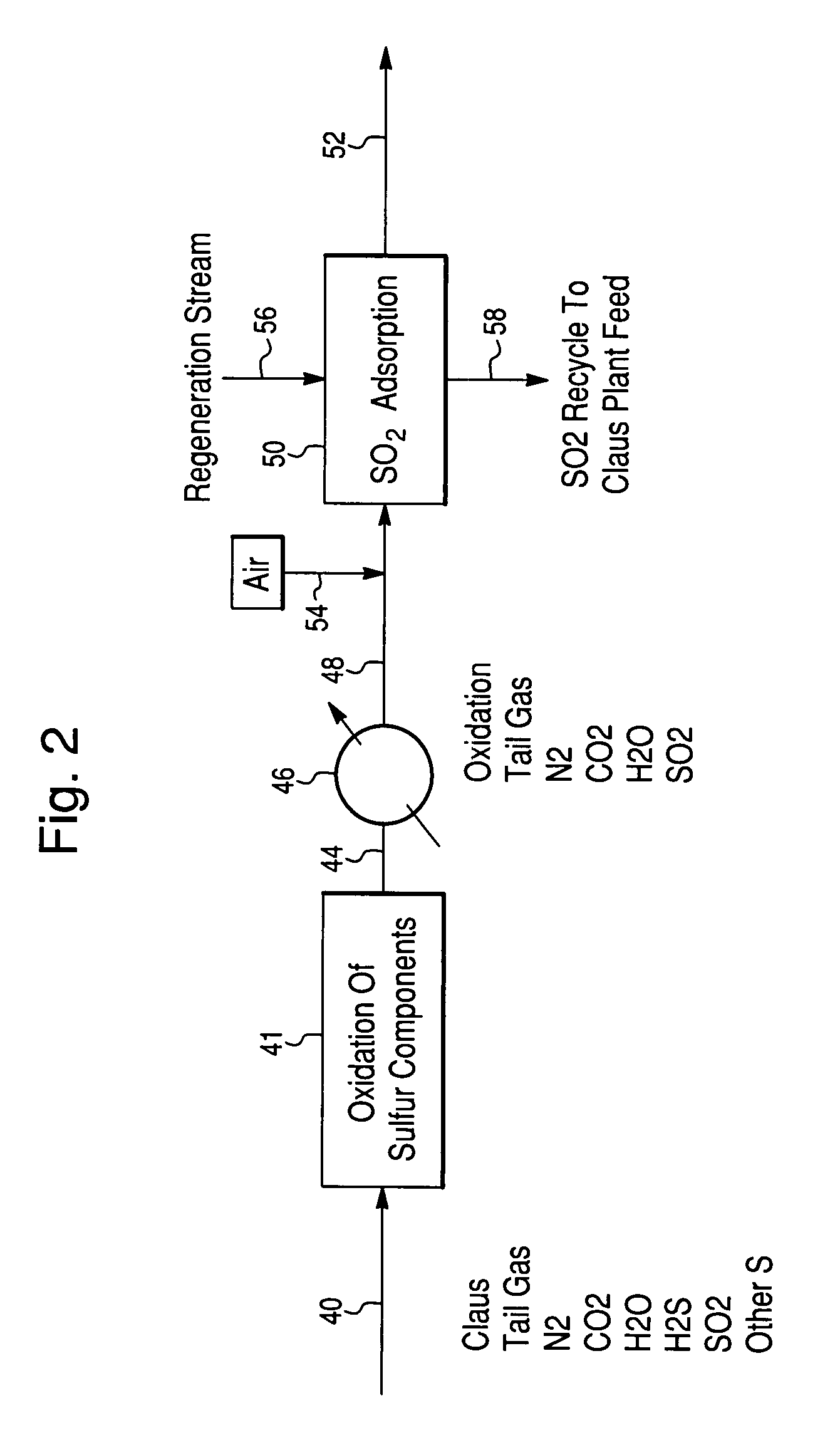
Process for the recovery of sulfur from Claus tail gas streams
a technology for tail gas streams and sulfur, applied in the direction of sulfur compounds, sulfur preparation/purification, separation processes, etc., can solve the problem that the shell scot process costs approximately 12 to 13 the cost of the claus plant itsel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0036]The proposed mechanism for the adsorption of SO2 on activated carbon in the presence of O2 and H2O is the formation of an adsorbed sulfuric acid species, which is then thermally regenerated / reduced back to SO2. To test this theory, two adsorbent samples were impregnated with sulfuric acid: (1) an activated carbon with 35% H2SO4 and (2) 1.9% Pt / ZSM-5 having a SiO2 / Al2O3 ratio of 270 with 20% H2SO4. Each acid loaded sample was placed in a column and then regenerated at 260° C. with wet N2. The SO2 / SO3 content of the off-gas was determined by wet analysis.
[0037]The loading for the activated carbon was 7.76 g (0.079 mol) of H2SO4 on 13.7 g of carbon. The SO2 / SO3 split upon regeneration was determined to be 4.91 g SO2 (0.077 mol) and 0.21 g of SO3 (0.002 mol). Remarkably, 100% recovery of SO2 / SO3 (0.079 mol) was achieved with the formation of only 4% of undesirable SO3 / H2SO4, a very favorable situation.
[0038]The loading for Pt / ZSM-5 was 6.76 g (0.069 mol) of H2SO4 on 25.6 g of adso...
example 2
[0039]This example compares the impact of the feed components during adsorption. SO2 adsorption was compared with and without O2 or H2O present in the fuel. Breakthrough times (detection of SO2 in exit gas) were normalized to 20.0 g:
[0040]Sample: 15.6 g (dry basis) of Norit®RO activated carbon (0.8 mm extrudates)
[0041]Adsorption Temp: 90° C.
[0042]Adsorption Pressure: 20 psia
[0043]Feed Flow: 73 sccm
[0044]Duplicate SO2 breakthrough tests on Norit®RO activated carbon using a feed stream containing 3,100 ppm SO2, ˜22% CO2, ˜22% H2O, balance N2 resulted in an average breakthrough time of 219 minutes. Results were significantly better with O2 present as shown next. Breakthrough tests were repeated using a feed stream containing 3,100 ppm SO2, 22% CO2, 9,000 ppm O2, ˜22% H2O, balance N2. In this case no breakthrough of SO2 was noted even after 2,880 minutes, the point at which the run was stopped. In the presence of O2, loading of SO2 was >11.9 wt % SO2 (g / g ads.) as compared to 0.9% wt % ...
example 3
[0047]In this example, the impact of inert gas regeneration of the adsorbent was studied.
Regeneration with N2 (9 cycle life test):
[0048]Sample: 14.6 g (dry basis) of Norit®RO activated carbon (0.8 mm extrudates)
[0049]Adsorption Temp: 90° C.
[0050]Adsorption Pressure: 20 psia
[0051]SO2 adsorption steps were run with a feed containing 5% SO2, 5% O2, 24% H2O, balance N2 at 90° C. The feed flow was adjusted to 73 sccm so as to achieve a less than four hour breakthrough time. Regeneration steps were carried out at 260° C. with wet helium at 73 cc / min of He with 1 ml / min H2O for three hours. The final hour of the regeneration cycle was used for cooling the bed. Significant CO2 was detected by the GC during regeneration. A GC scan of the regeneration off-gas from the 8th cycle showed that the production of CO2 was directly associated with the release of SO2. Integration of the peaks indicated a ˜2.6 / 1 SO2 / CO2 molar ratio. This ratio is consistent with carbon oxidation by the adsorbed sulfuri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com