System and method for tissue specific MR imaging of metabolites using spectral-spatially formed stimulated echo
a tissue specific and stimulated echo technology, applied in the field of magnetic resonance imaging, can solve problems such as indistinct csi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
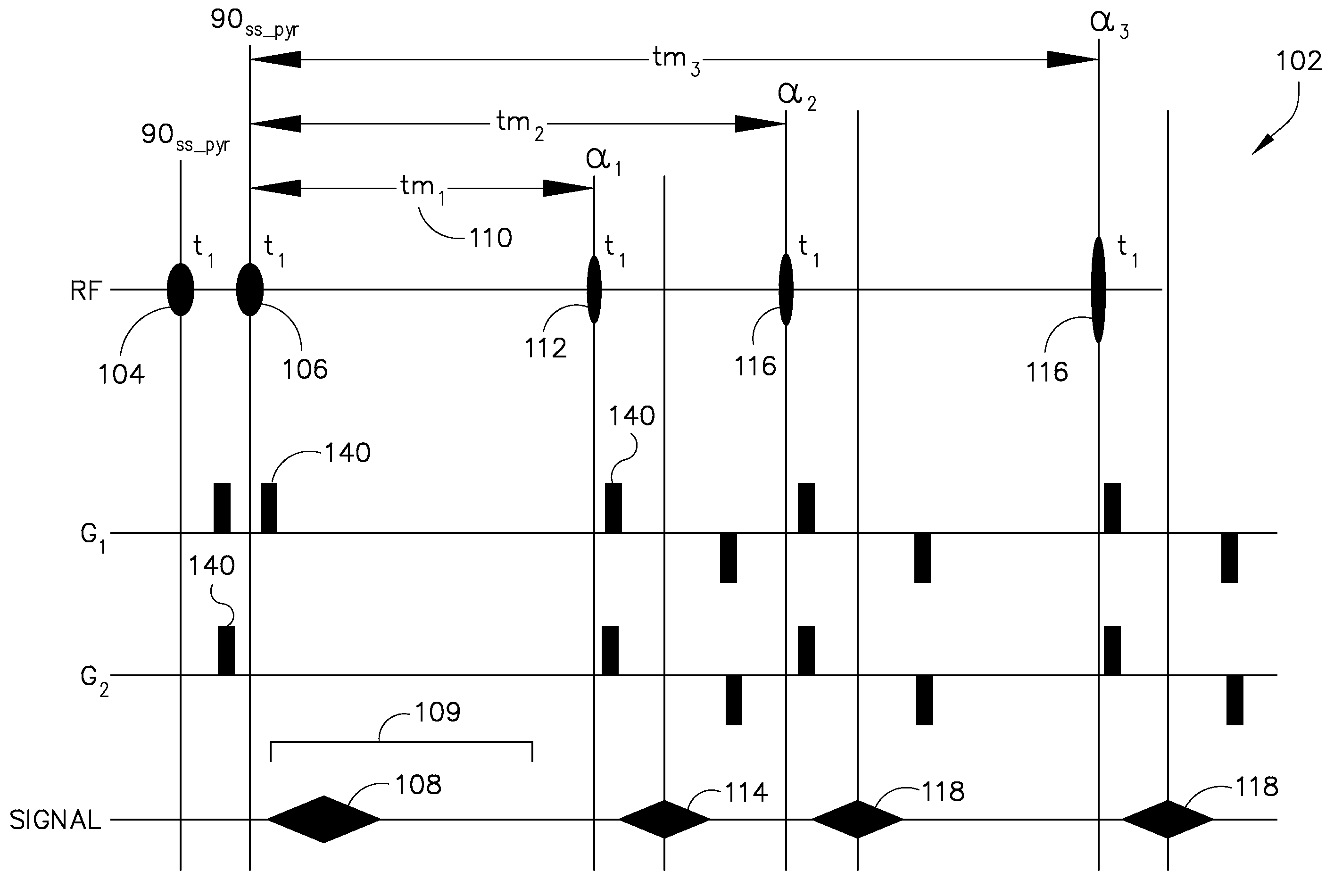
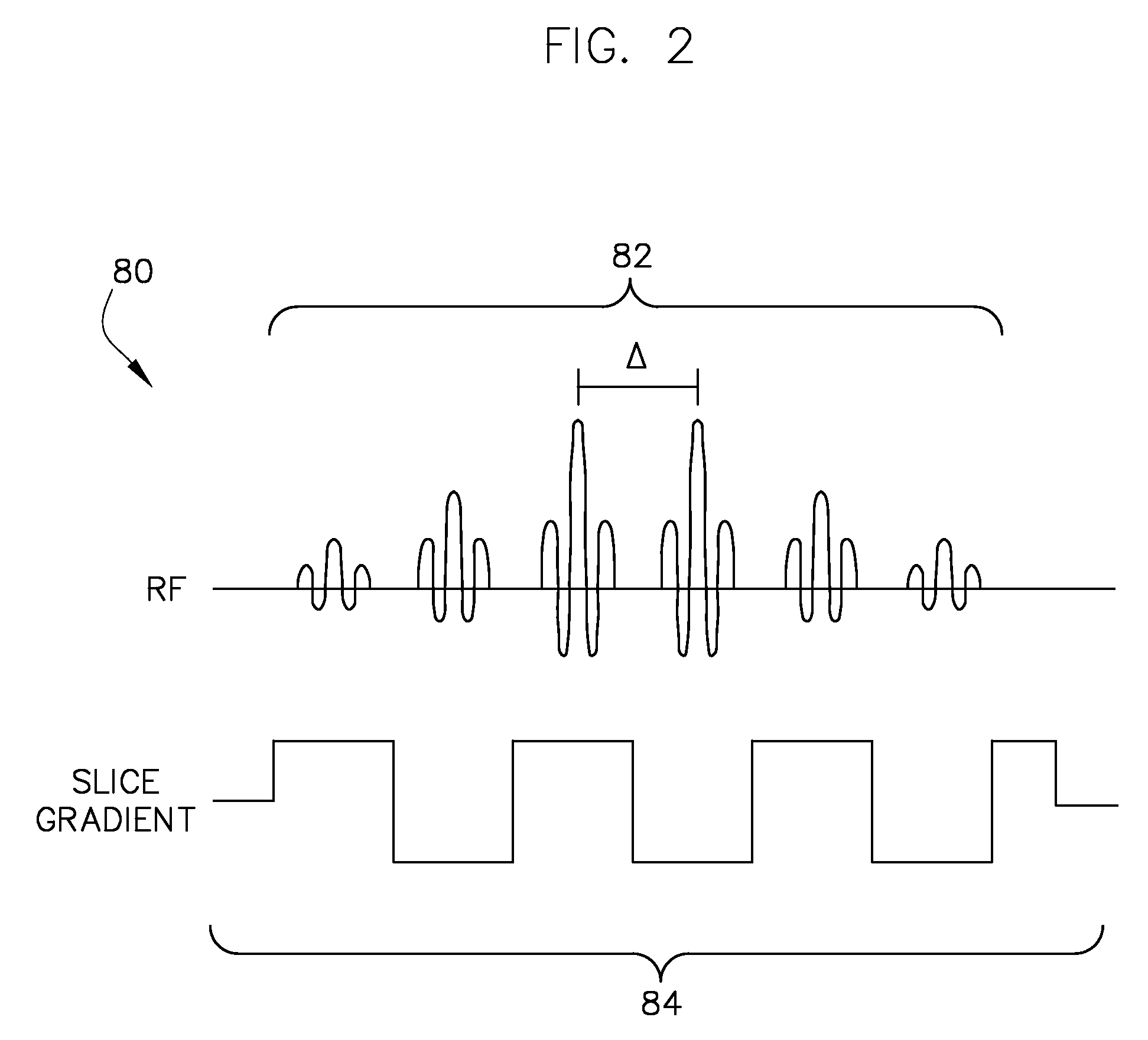
[0021]A system and method for slice-selectively exciting a resonant frequency for a metabolic reactant and for reading out a resulting metabolic process as a spectroscopic image is provided. In this regard, a pair of RF pulses may be emitted to spectral-spatially excite a frequency in a subject of interest for a specified metabolic reactant. Spectral-spatial radio frequency (RF) pulses may be used to create magnetization in a specific frequency profile without significantly affecting neighboring slices or nearby frequency ranges. A third RF pulse (either non-spectrally selective or spectrally selective) may then be emitted to generate a stimulated echo and excite a broad range of frequencies in the subject of interest for a plurality of metabolic products derived in vivo from the metabolic reactant. The signals resulting from these pulses are read in one or more of several well-known read out sequences.
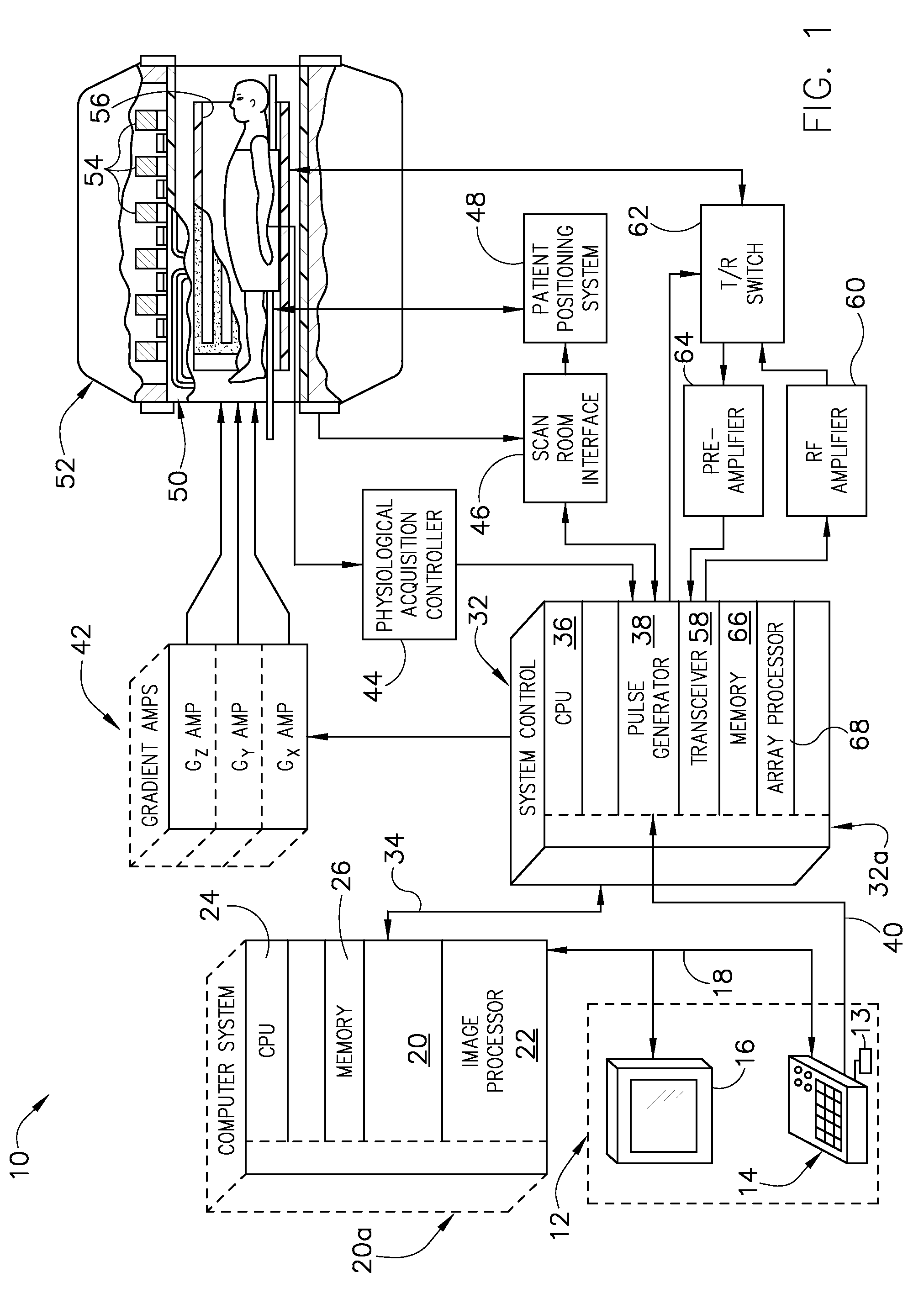
[0022]Referring to FIG. 1, the major components of an exemplary magnetic resonanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com