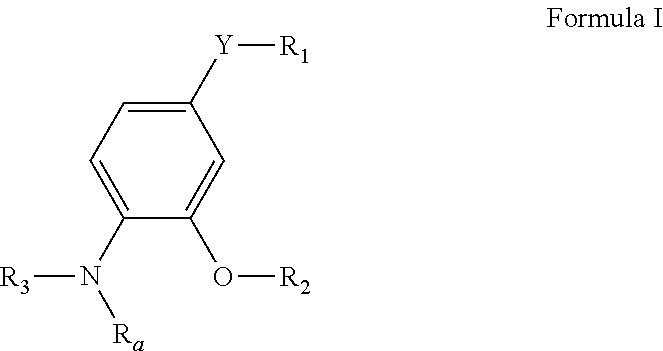
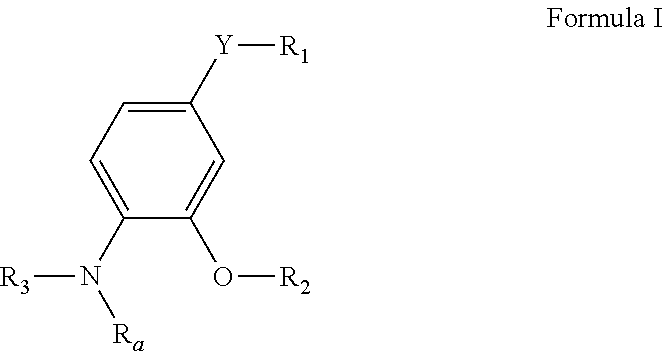
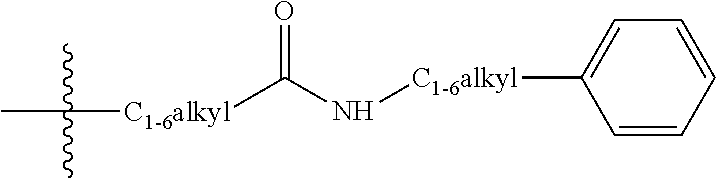
4-substituted-2-phenoxy-phenylamine delta opioid receptor modulators
a technology of opioid receptor and phenylamine, which is applied in the field of opioid receptor modulators, can solve the problems of difficulty in mention, inability to concentrate, and experience not always pleasan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 7
[0379]
[0380]A. (2S,4R)-4-Fluoro-2-(methoxy-methyl-carbamoyl)-pyrrolidine-1-carboxylic acid tert-butyl ester (7b). HBTU (12.3 g, 32.3 mmol) was added in portions to a solution of Compound 7a (6.26 g, 26.9 mmol), N,O-dimethylhydroxylamine hydrochloride (3.15 g, 32.3 mmol), and DIEA (5.62 mL, 4.17 g, 32.3 mmol) in 60 mL of DMF at 0° C. After 15 min, the cooling bath was removed and the mixture was stirred 16 h at 20° C. EtOAc (200 mL) and saturated aqueous NH4Cl (100 mL) were added. The organic layer was separated, washed with saturated aqueous NaHCO3 (100 mL), and brine (100 mL), and dried over MgSO4. The solution was concentrated to give 7.7 g of off-white oil that was purified by flash column chromatography (SiO2), eluting with 10% MeOH / CH2Cl2, to yield Compound 7b (5.47 g, 74% yield). 1H-NMR (DMSO-d6): δ 5.29 (1H, dt), 4.70 (1H, dd), 3.50 (2H, m), 3.13 (3H, s), 2.69 (3H, s),2.00 (2H, m), 1.34 (9H, s).
[0381]B. (2S,4R)-4-Fluoro-2-formyl-pyrrolidine-1-carboxylic acid tert-butyl ester ...
example 8
[0382]
[0383]A. (2S,4R)-2-{[4-Bromo-2-(4-methoxy-phenoxy)-phenylamino]-methyl}-4-fluoro-pyrrolidine-1-carboxylic acid tert-butyl ester (8a). A mixture of Compound 1d (1.18 g, 4.0 mmol), Compound 7c (1.33 g, 6.1 mmol), and 5 drops of HOAc in 10 mL of DCE was stirred for 5 min at 20° C. NaBH(OAc)3 (2.54 g, 12 mmol) was added in portions over a 5 min period and the mixture was stirred at 20° C. for 20 h. Saturated aqueous NH4Cl (25 mL) was added to the stirring mixture, followed by 100 mL of CH2Cl2 and 10 mL of water. The organic layer was separated and the aqueous layer was extracted with 50 mL of CH2Cl2. The combined organic layers were dried over Na2SO4 and concentrated to give 2 g of crude product. This material was purified by flash column chromatography (SiO2), eluting with a hexanes-EtOAc gradient, to yield Compound 8a (0.496 g, 25% yield). MS: m / z 495.0 / 497.0 (M+H)+.
[0384]B. (2S,4R)-2-{[4-(5-Cyano-pyridin-3-yl)-2-(4-methoxy-phenoxy)-phenylamino]-methyl}-4-fluoro-pyrrolidine-1-ca...
example 9
[0388]
[0389]A. 2-(S)-{[3-(4-Carbamoyl-phenoxy)-biphenyl-4-ylamino]-methyl}-pyrrolidine-1-carboxylic acid tert-butyl ester (9a). 20% aqueous NaOH (0.5 mL) and 30% aqueous H2O2 (0.4 mL) were added to a solution of compound 6f (0.040 g, 0.085 mmol) in 1.5 mL of EtOH and 1.5 mL of dioxane. The mixture was heated at 60° C. for 2 days. The reaction mixture was extracted with EtOAc, dried over MgSO4, and concentrated to give Compound 9a. MS: m / z 488.3 (M+H)+.
[0390]B. Cpd 29: (S)-4-{4-[(Pyrrolidin-2-ylmethyl)-amino]-biphenyl-3-yloxy}-benzamide. A mixture of Compound 9a, TFA, and CH2Cl2 was stirred at 20° C. for 2 h. After concentration, the residue was dissolved in CH3CN and purified by reverse phase HPLC to afford Cpd 29 as a TFA salt (0.015 g, 29% yield for 2 steps). 1H NMR (300 MHz, CD3OD): δ 7.87-7.89 (m, 2H), 7.33-7.49 (m, 5H), 7.18-7.19 (m, 2H), 7.00-7.06 (m, 3H), 3.84-3.95 (m, 1H), 3.44-3.57 (m, 2H), 3.28-3.35 (m, 2H), 1.98-2.28 (m, 3H), 1.72-1.82 (m, 1H); MS: m / z 387.9 (M+H)+.
[0391]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com