Prep. of epsilon-hexanolactam
A technology of caprolactam and cyclohexanone oxime, applied in the field of ε-caprolactam preparation, can solve the problem of high equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
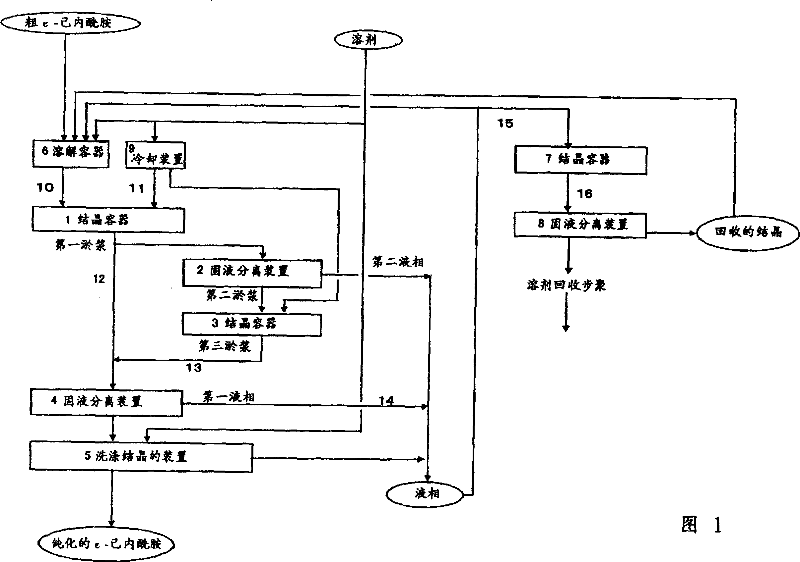
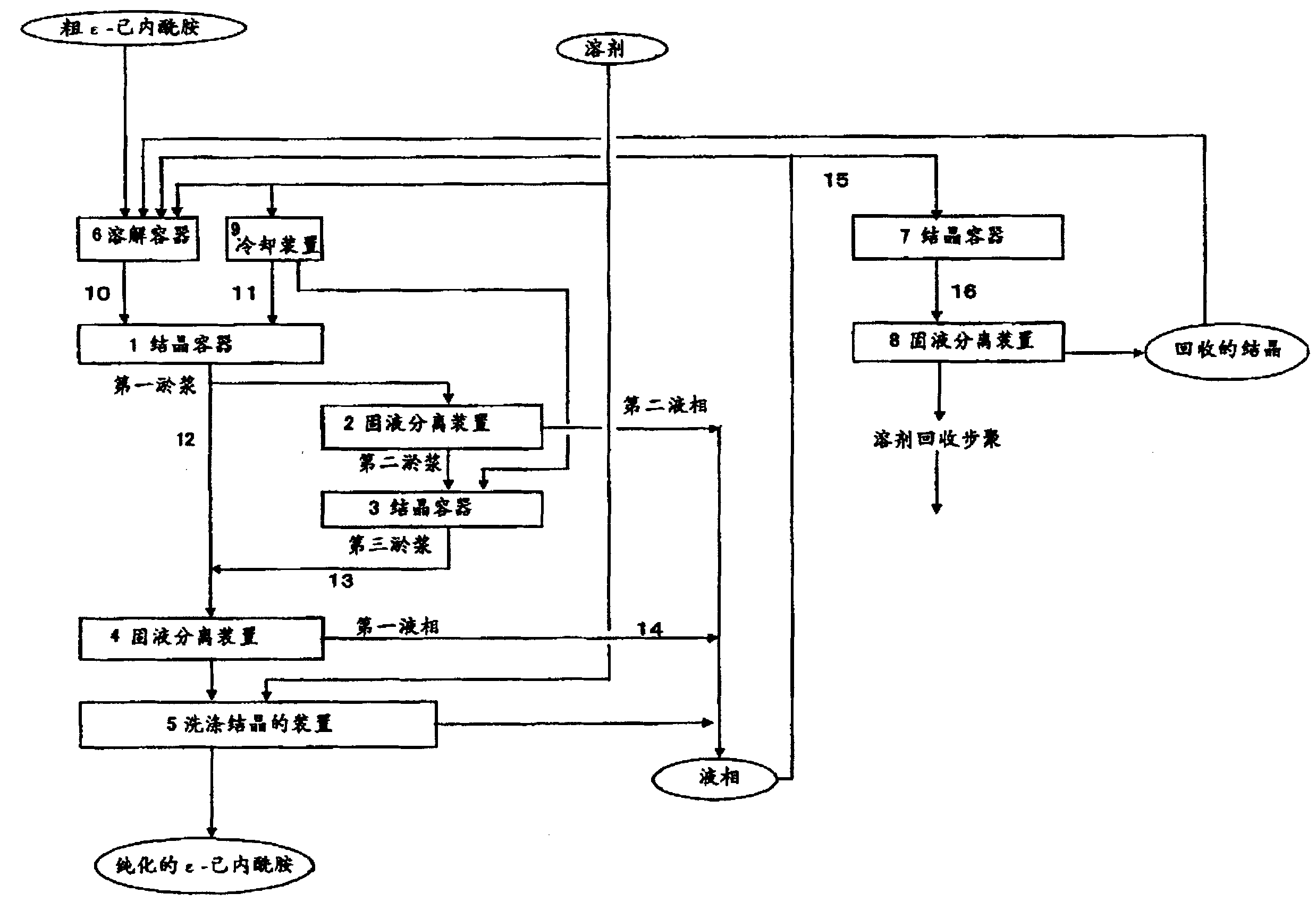
[0042] Referring to Fig. 1, an embodiment of the method of the present invention, a method for continuously producing ε-caprolactam, is described below. In Fig. 1, reference numeral 1, 3 and 7 all represent crystallization vessel, 2, 4 and 8 represent solid-liquid separation device, 5 represent the device of washing crystallization, 6 represent dissolution vessel, 9 represent cooling device, 10-16 Represents a pipeline.
[0043] The molten crude ε-caprolactam prepared in dissolution vessel 6 enters crystallization vessel 1 via line 10 . At the same time, the solvent cooled in the cooling device 9 enters the crystallization vessel 1 through the line 11 . In the preparation of the molten crude ε-caprolactam in the dissolution vessel 6, solvents including aliphatic hydrocarbons may be used. At this time, the obtained ε-caprolactam contains a small amount of cyclohexanone oxime compared to the case where no aliphatic hydrocarbon-containing solvent is used in the dissolution vess...
Embodiment 1
[0069] The process for the continuous production of ε-caprolactam is carried out as follows. Flow rates of liquids are expressed in parts by weight per unit of time (unless otherwise stated).
[0070] A gas-phase Beckmann rearrangement reaction of cyclohexanone oxime (hereinafter referred to as "OXM") was carried out at 380°C in the presence of methanol using a fluidized bed reactor packed with a high-silica zeolite catalyst to obtain crude ε-caprolactam-containing reaction mixture.
[0071] The reaction mixture was distilled to remove methanol, low-boiling impurities and high-boiling impurities to obtain crude ε-caprolactam with a purity of 99.131% containing 13 ppm OXM, 398 ppm MTHI and 430 ppm OHP.
[0072] The crude ε-caprolactam (200 parts by weight; 75 ℃) and the mixed solvent (400 parts by weight; 5 ℃) of cyclohexane and n-heptane (weight ratio 1:3) obtained above and melted in advance were continuously poured into the The jacket keeps the crystallization vessel at 5...
Embodiment 2
[0081] A liquid mixture of OXM, methanol and water (weight ratio 1:1.8:0.052) was injected into a fluidized bed reactor packed with high silica zeolite catalyst through an evaporator, and the reaction temperature was 380 °C and the residence time was 8 The Beckmann rearrangement reaction of OXM was carried out under the conditions of seconds to obtain a reaction mixture containing crude ε-caprolactam.
[0082]The reaction mixture was distilled to remove methanol, low-boiling impurities, and high-boiling impurities to obtain crude ε-caprolactam with a purity of 99.08% containing 188 ppm OXM, 469 pm MTHI and 205 ppm OHP.
[0083] A mixture of the resulting crude ε-caprolactam (55 g) and n-heptane (82.5 g) was prepared and maintained at 70°C. Separately, n-heptane (41.25 g) was cooled with ice. The mixture of crude ε-caprolactam and n-heptane was continuously poured (over 10 minutes) into a flask filled with other n-heptane (41.25 g) together with cooled n-heptane at a temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com