Abedol slow release tablet and its preparation method
A technology of Arbidol and sustained-release tablets, which is applied in the field of antiviral drug Arbidol hydrochloride sustained-release tablets, which can solve the problems of inconvenient medication and short half-life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
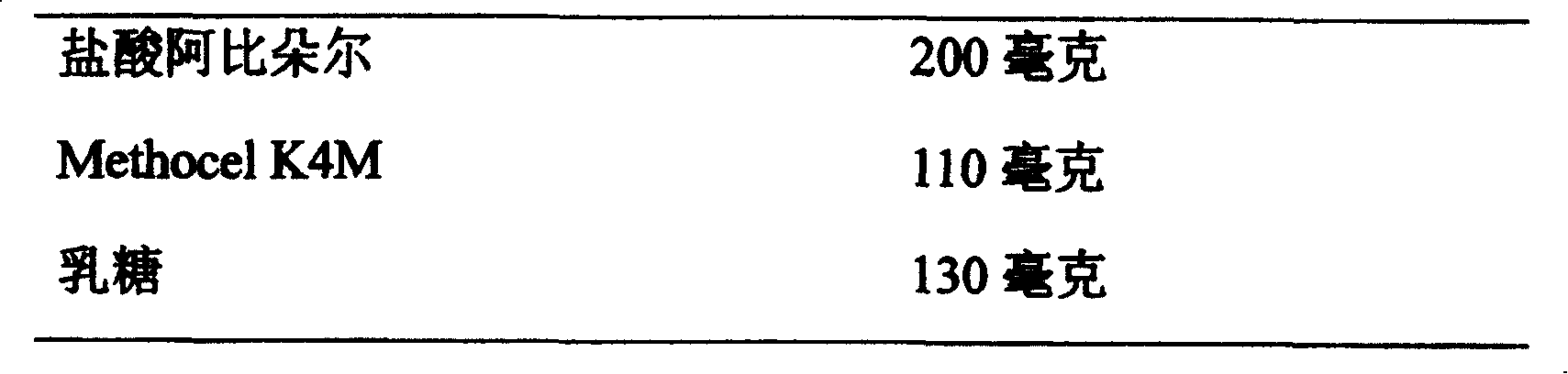
[0039] Example 1: Arbidol Hydrochloride Hydrophilic Gel Matrix Sustained Release Tablets
[0040] formula:
[0041]
[0042]
[0043] Preparation Process:
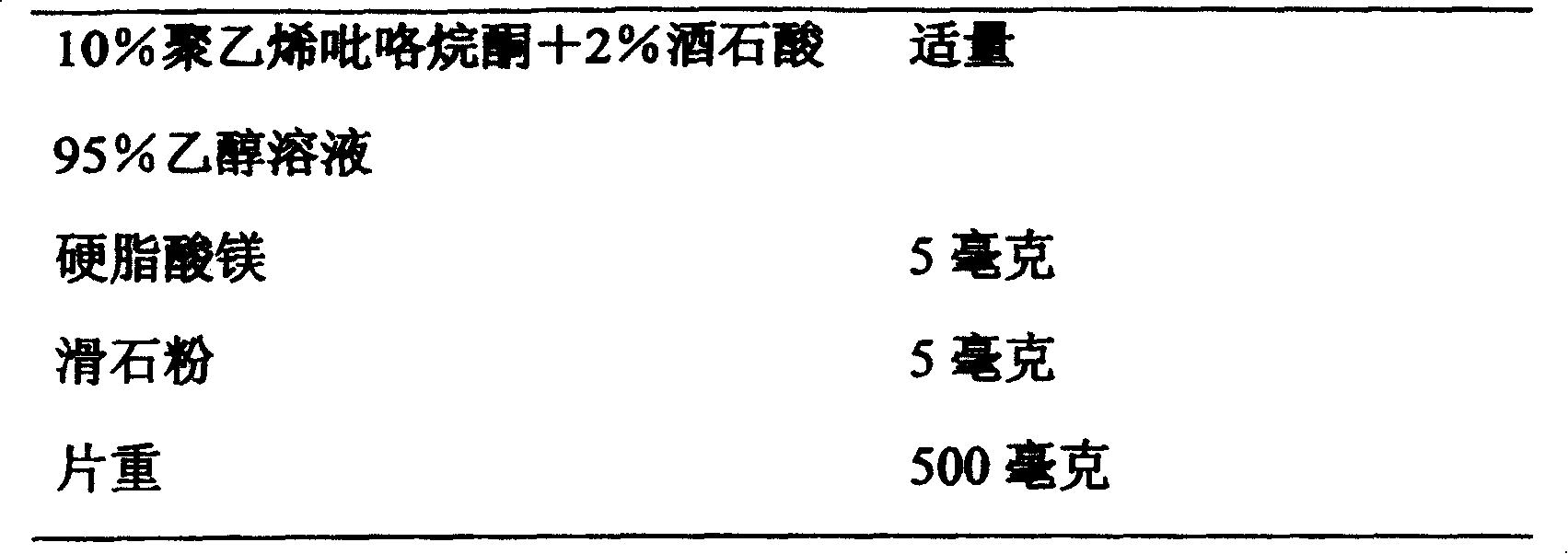
[0044] Grind Arbidol hydrochloride through a 80-mesh sieve, mix evenly with Methocel K4M and lactose, moisten with 10% polyvinylpyrrolidone + 2% tartaric acid 95% ethanol solution, then granulate, dry at 45-50°C, and granulate. Add magnesium stearate and talcum powder, mix evenly and press into tablets.
[0045] Release test:
[0046] In order to investigate the in vitro release characteristics of Arbidol Hydrochloride Sustained-release Tablets, the release of the samples was determined by the first method (basket method) of Appendix XC of Part Two of the Chinese Pharmacopoeia 2000 Edition. With 900ml of water as solvent, the rotating speed is 100 revolutions per minute, sampling respectively in 1, 2, 4, 6, and 8 hours, according to spectrophotometry (Chinese Pharmacopoeia 2000 edition two appendix IVA), measure t...
Embodiment 2
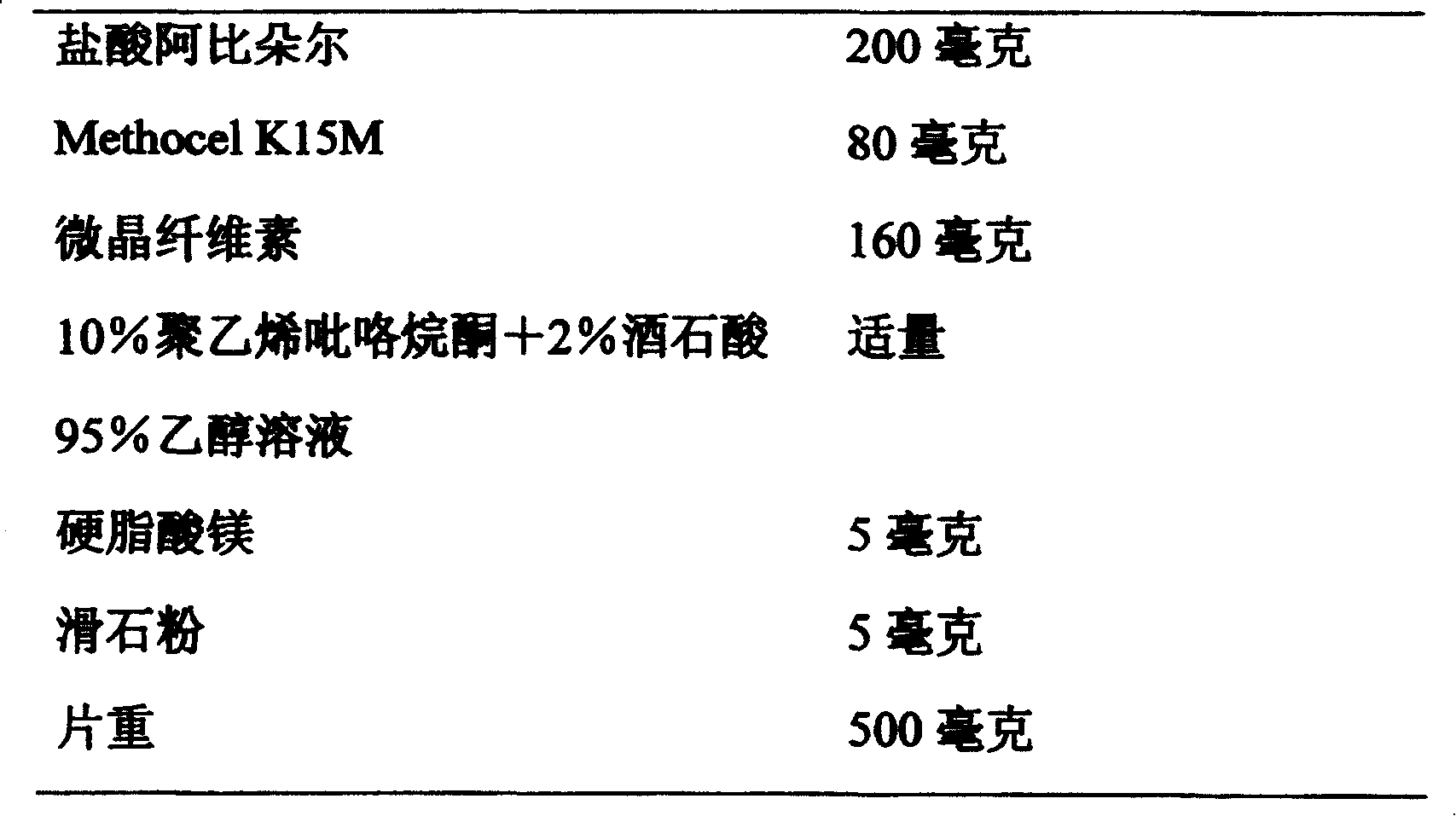
[0051] Embodiment 2: Arbidol Hydrochloride Hydrophilic Gel Matrix Sustained Release Tablets
[0052] formula:
[0053]
[0054] Preparation Process:
[0055] Grind Arbidol hydrochloride through an 80-mesh sieve, mix it with Methocel K15M and microcrystalline cellulose, wet it with 10% polyvinylpyrrolidone + 2% tartaric acid 95% ethanol solution, and granulate it, and dry it at 45-50°C Whole grains, add magnesium stearate and talcum powder, mix evenly and press into tablets.
Embodiment 3
[0056]Embodiment 3: Arbidol Hydrochloride Hydrophilic Gel Matrix Sustained Release Tablets
[0057]
[0058]
[0059] Preparation Process:
[0060] Arbidol hydrochloride was crushed through a 80-mesh sieve, mixed evenly with sodium alginate and microcrystalline cellulose, then wetted with 0.5% hydroxypropyl methylcellulose + 2% tartaric acid 60% ethanol solution, and then granulated, at 45 ~50□ After drying, granulate, add magnesium stearate and talcum powder, mix evenly and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com