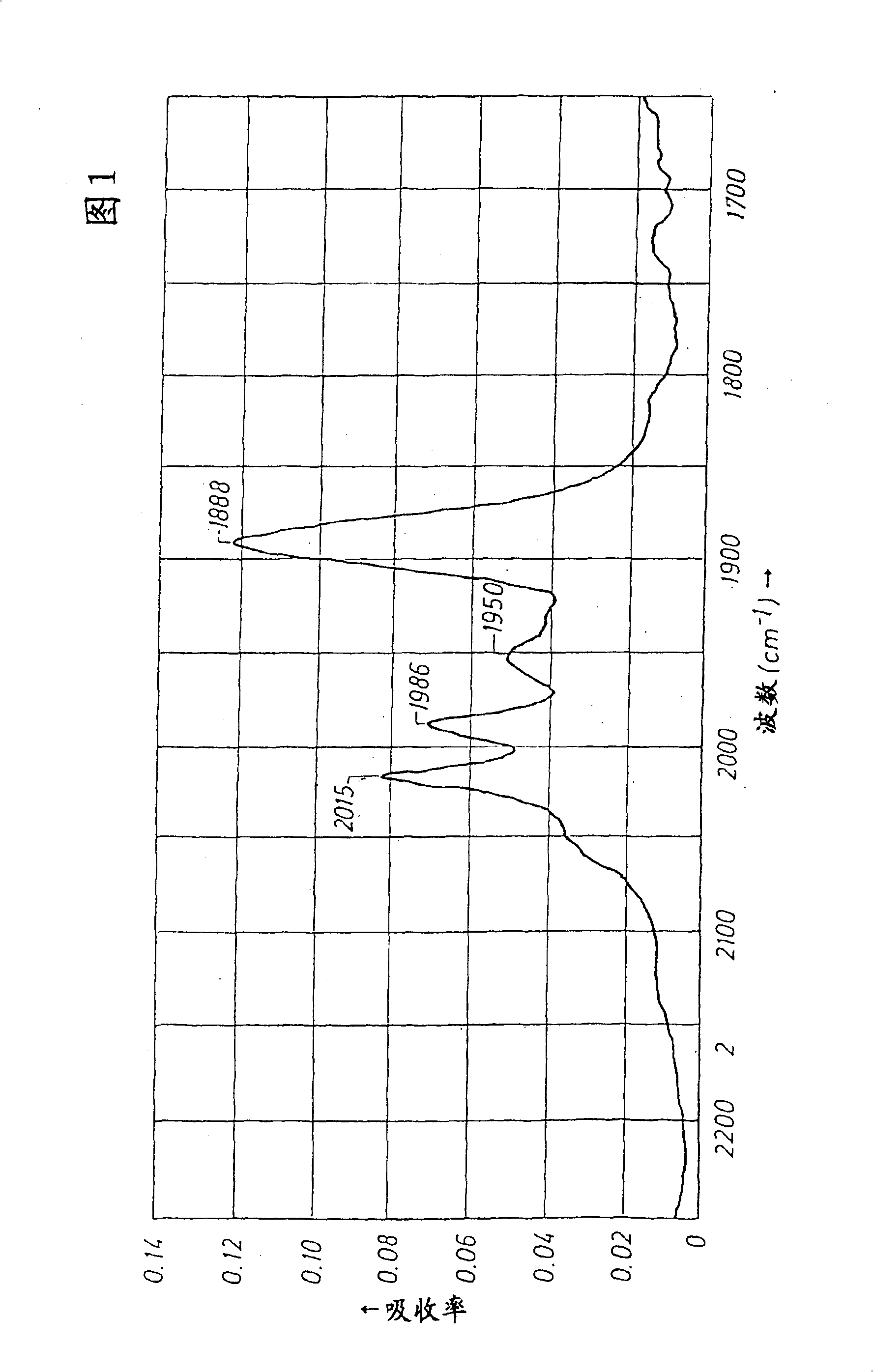
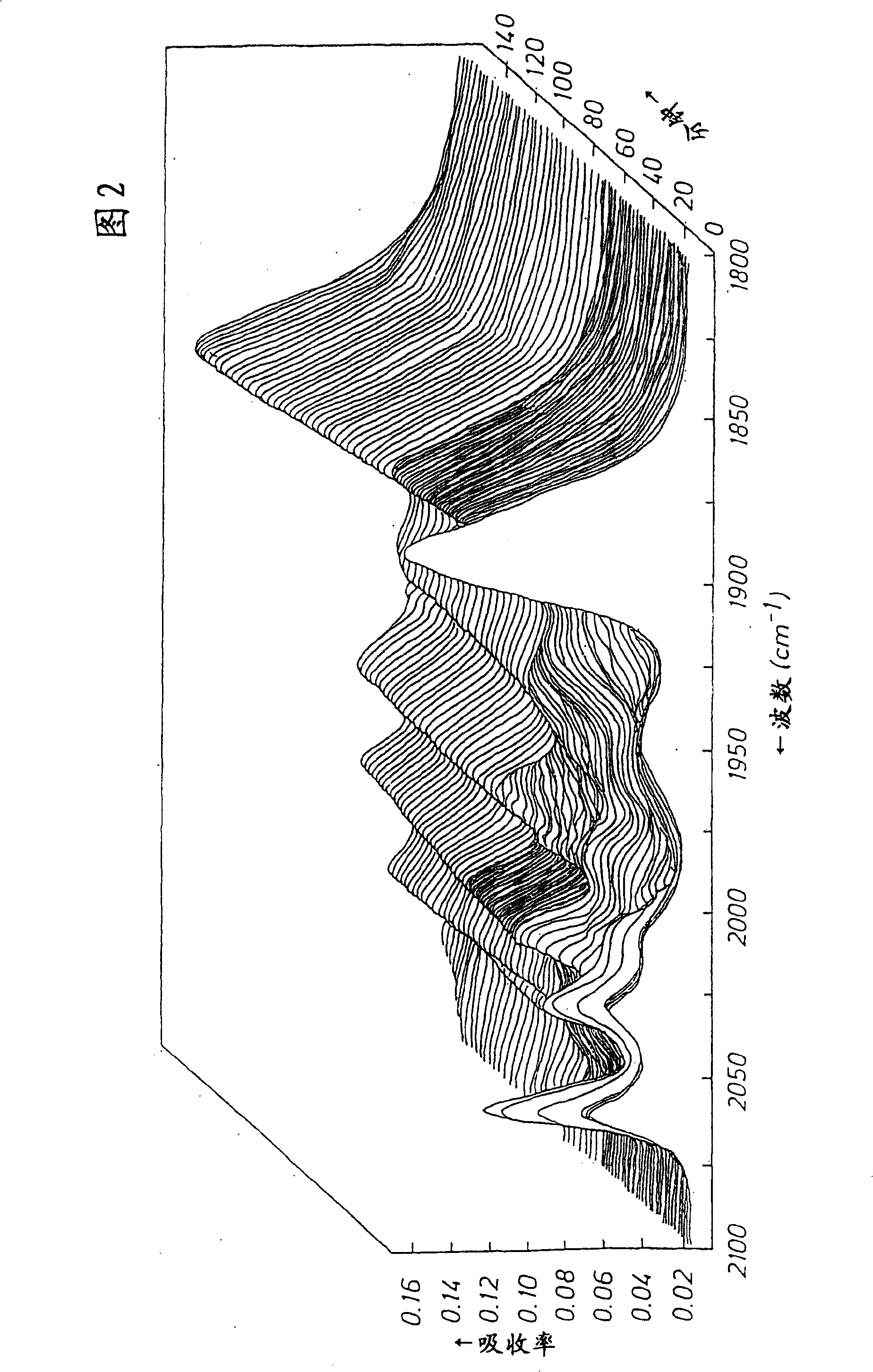
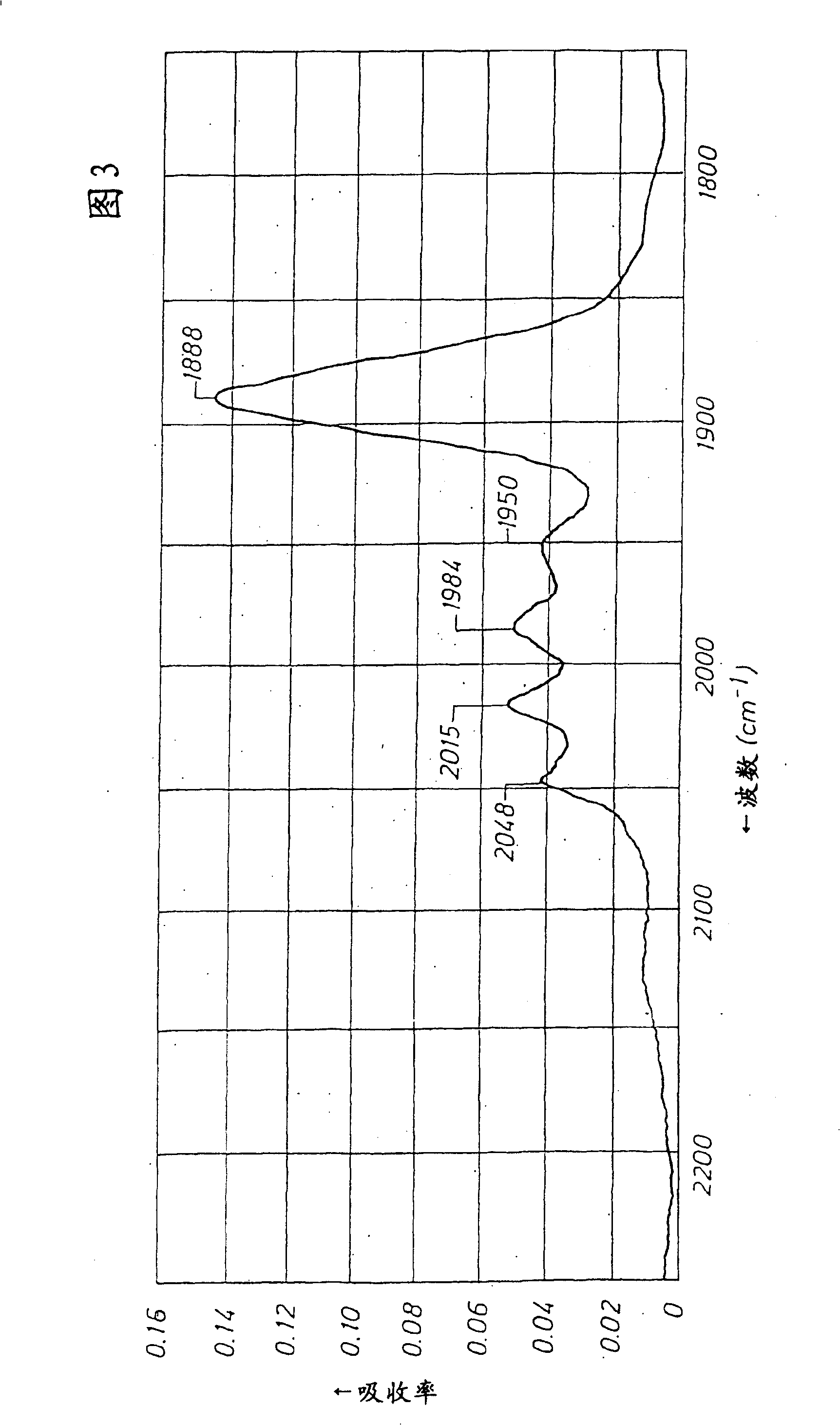
One-step production of 1,3-propanediol from ethylene oxide and syngas with a catalyst with a N-heterocyclic ligand
A catalyst and heterocycle technology, applied in the field of one-step production of 1,3-propanediol from ethylene oxide and synthesis gas using catalysts with N-heterocycle ligands, which can solve the problem of expensive phosphine ligands and achieve good oxidation The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-20
[0069] Examples 1-20 were carried out in a 300 cc capacity Parr reactor system connected to a syngas manifold. In Examples 1-12 the N-heterocyclic ligand was changed but only two cyclic ether solvents were used. The solvents were varied in Examples 13-20. Changes to other components and conditions are noted in the notes. The test data are given in Tables 1 and 2.
[0070] As mentioned above, 2,2'-bipyridine (DIPY), 2,2'-bipyrimidine (BPYM) and 2,4,6-tripyridyl-s-triazine (TPTZ) work particularly well. Table 1 presents the data using these three N-heterocycles and 1,10-phenanthroline (PHEN) in the one-step PDO synthesis. Wherein the PDO yield is calculated in moles based on the amount of ethylene oxide added, and the selectivity of PDO is estimated by gas chromatography (GC) analysis of the crude product fraction. Major by-products include ethanol (major by-product fraction), HPA intermediates, acetaldehyde and small amounts including 3-hydroxypropyl-2-hydroxyethyl ether, 3...
Embodiment 21
[0076] A typical lifetime study of the catalyst complex was performed in Example 21. The octacarbonyl dicobalt-dodecacarbonyl triruthenium-2,2'-bipyridine catalyst dissolved in 1,3-dioxolane was used as the catalyst precursor, EO was added 18 times and PDO distillation was carried out 4 times. Where the initial Co-Ru-DIPY stoichiometric ratio is 1:1:1, each EO hydroformylation is at 1 / 4 (CO / H 2 ) under syngas. Typical steps are as follows:
[0077] 1. Four additions of EO to a Co-Ru-N-heterocyclic catalyst in a cyclic ether solvent, each addition of EO was converted to PDO by hydroformylation / hydrogenation as described above.
[0078] 2. After removing the solvent, recover PDO by vacuum distillation.
[0079] 3. The bottom Co-Ru-N-heterocyclic catalyst solution in the PDO is circulated with fresh ether solvent.
[0080] The data are shown in Table 3:
[0081] table 3
[0082]
[0083] In general, a slow accumulation of organic heavy components, especially 3-hyd...
Embodiment 22
[0085] A series of experiments very similar to Example 21 was also performed in which intermediate solids formed during multiple cycles were removed by filtration (before distillation of PDO) and a small amount of make-up catalyst was added after 18 additions of EO. Add 4 more EOs for a total of 22. The EO uptake times for this second catalyst lifetime study are shown in FIG. 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com