Folid acid derivatives and their salts for preparing antitumor medicine
A technology of folic acid derivatives and drugs, applied in the fields of chemistry and medicine, can solve the problems of solid tumors with little effect, unsatisfactory treatment effect, toxicity, etc., and achieve the effect of overcoming drug resistance and strong growth inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
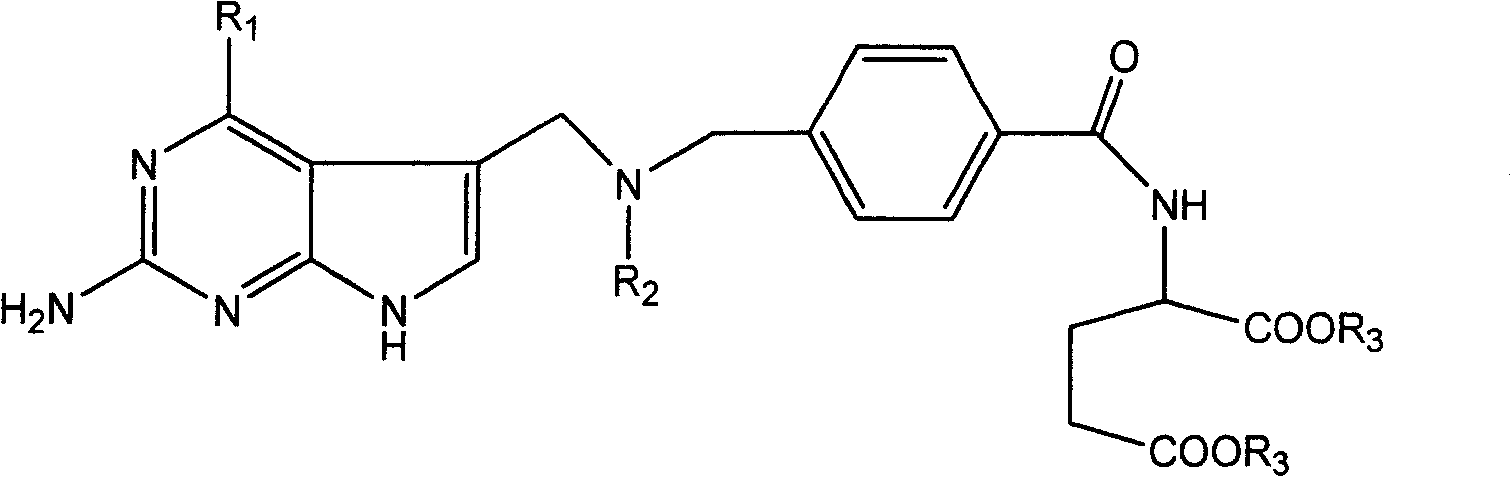
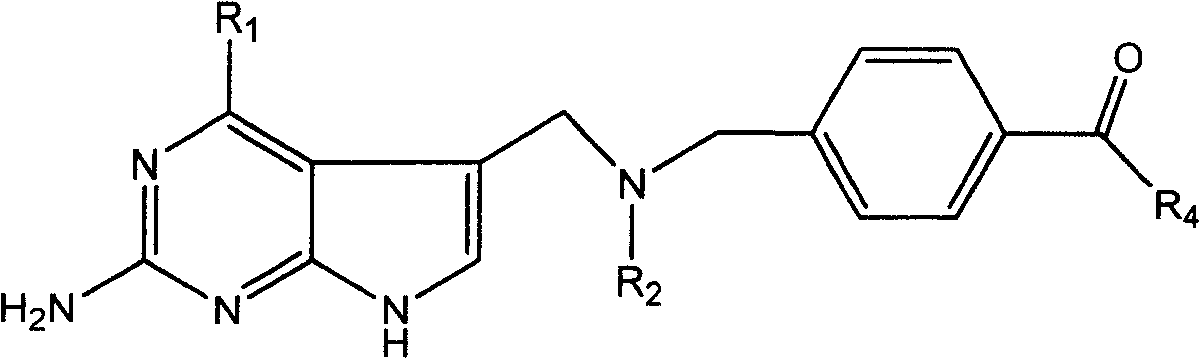
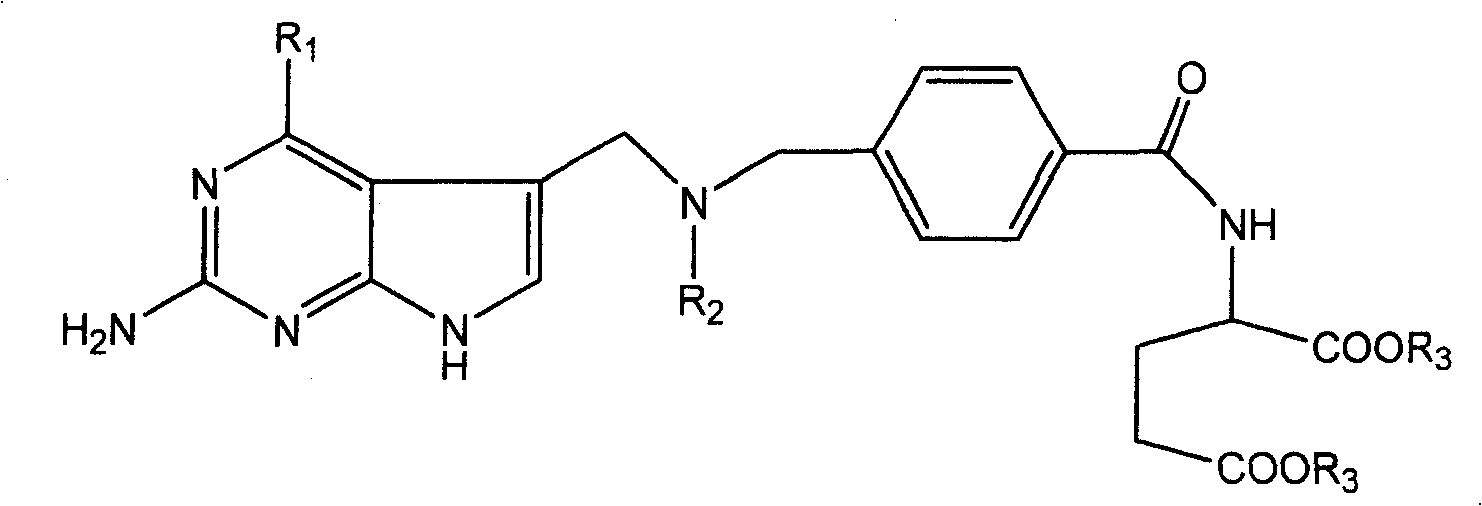
[0031] This type of folic acid derivatives and salts thereof of the present invention can be synthesized by various methods, hereinafter referred to as R 1 =NH 2 , R 2 =CH 3 and CH 2 CH 3 As an example, to illustrate the synthetic route of the present invention, but not limited to this functional group.
[0032] step 1
[0033] Synthesis of 2,4-diamino-5-cyano-7hydro-pyrrolo[2,3-d]pyrimidine (1)
[0034] Reaction formula:
[0035]
[0036] Sodium acetate (13.1 g, 96 mmol) was added to 180 ml of distilled water, and then 2,4,6-triaminopyrimidine (6.0 g, 48 mmol) was added, heated to 50° C., and the solid dissolved. Maintaining the temperature at 50°C, 2-chloro-3-oxo-propionitrile solution (40ml) was slowly added dropwise over 1.5h. The solution turned from colorless to orange-red and then gray-brown. Stirring and reaction were continued at this temperature for 12 hours, the remaining THF in the solution was distilled off under reduced pressure, and then the temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com