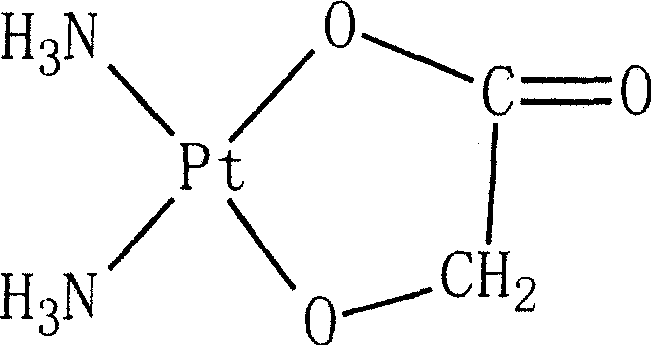
Preparation of nedaplatin freeze-dried powder injection
A technology of freeze-dried powder injection and nedaplatin, which is applied in the direction of freeze-drying delivery, powder delivery, active ingredients of anhydride/acid/halide, etc. It can solve problems such as difficult water sublimation, gray color, and prolonged freeze-drying time , to achieve the effect of shortening the freeze-drying time, reducing the scrap rate and shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of nedaplatin freeze-dried powder injection (10mg specification) by common method
[0019] prescription
[0020]
[0021]
[0022] Take the prescription amount of nedaplatin and put it in a container, add 80% water for injection, stir to dissolve and mix evenly, then add the prescription amount of dextran, stir to dissolve and mix evenly, measure the content of the intermediate, and add water for injection after passing the test Make up to the full volume, filter it with a 0.22 μm microporous membrane until it becomes clear under aseptic conditions, fill the filtrate into a sterile vial, partially plug a butyl rubber stopper, put it on a plate, and send it to a freeze dryer. Close the door of the box, open the freeze dryer, use the heat-conducting oil to refrigerate the plate layer, so that the product temperature drops, when the sample reaches the eutectic point (about -10 °C), continue to freeze until the product temperature is lower than -25 °C, sto...
Embodiment 2
[0024] Preparation of nedaplatin freeze-dried powder injection (50mg specification) by common method
[0025] prescription
[0026]
[0027] Take the prescription amount of nedaplatin and put it in a container, add 80% water for injection, stir to dissolve and mix evenly, then add the prescription amount of dextran, stir to dissolve and mix evenly, measure the content of the intermediate, and add water for injection after passing the test Make up to the full volume, filter it with a 0.22 μm microporous membrane until it becomes clear under aseptic conditions, fill the filtrate into a sterile vial, partially plug a butyl rubber stopper, put it on a plate, and send it to a freeze dryer. Close the door of the box, open the freeze dryer, use the heat-conducting oil to refrigerate the plate layer, so that the product temperature drops, when the sample reaches the eutectic point (about -10 °C), continue to freeze until the product temperature is lower than -25 °C, stop the plate ...
Embodiment 3
[0029]
[0030] Take the prescription amount of nedaplatin and put it in a container, add 80% water for injection, stir to dissolve it and mix it evenly, then add the prescription amount of dextran, stir it to dissolve and mix it evenly, measure the content of the intermediate, and add the prescription amount after passing the test. Stir the ethanol to make the mixture evenly, and then make up to the full volume with water for injection. Under aseptic conditions, filter it with a 0.22 μm microporous membrane until it becomes clear. , put it on a plate, put it into the freeze dryer, close the door, open the freeze dryer, use the heat-conducting oil to refrigerate the plate layer, so that the product temperature drops, when the sample reaches the eutectic point (about -10 ℃), continue to freeze to the product temperature When the temperature is lower than -25℃, stop the plate cooling and open the condenser. When the temperature of the condenser reaches -40℃, open the vacuum sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com