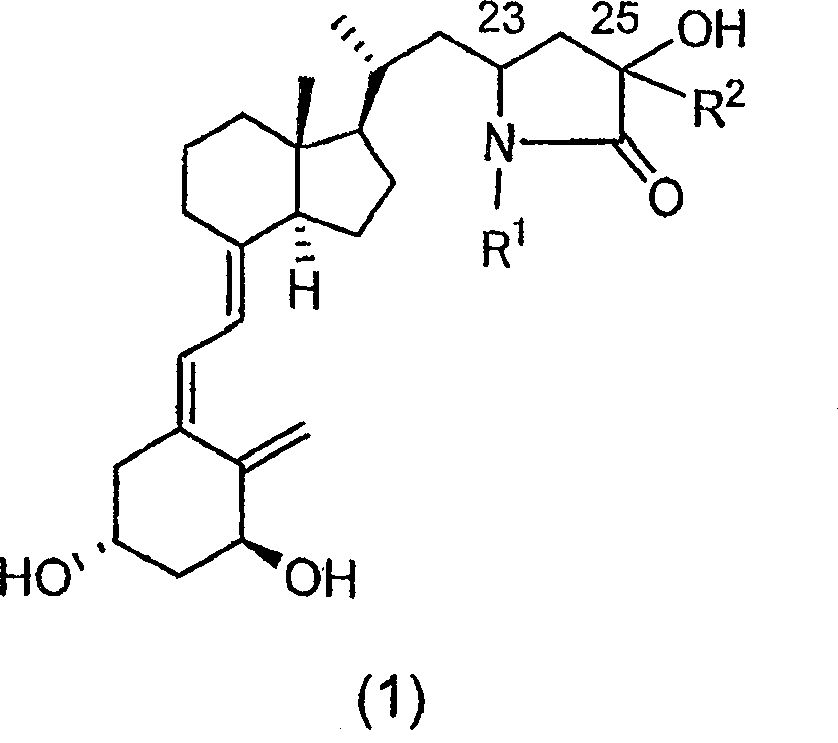
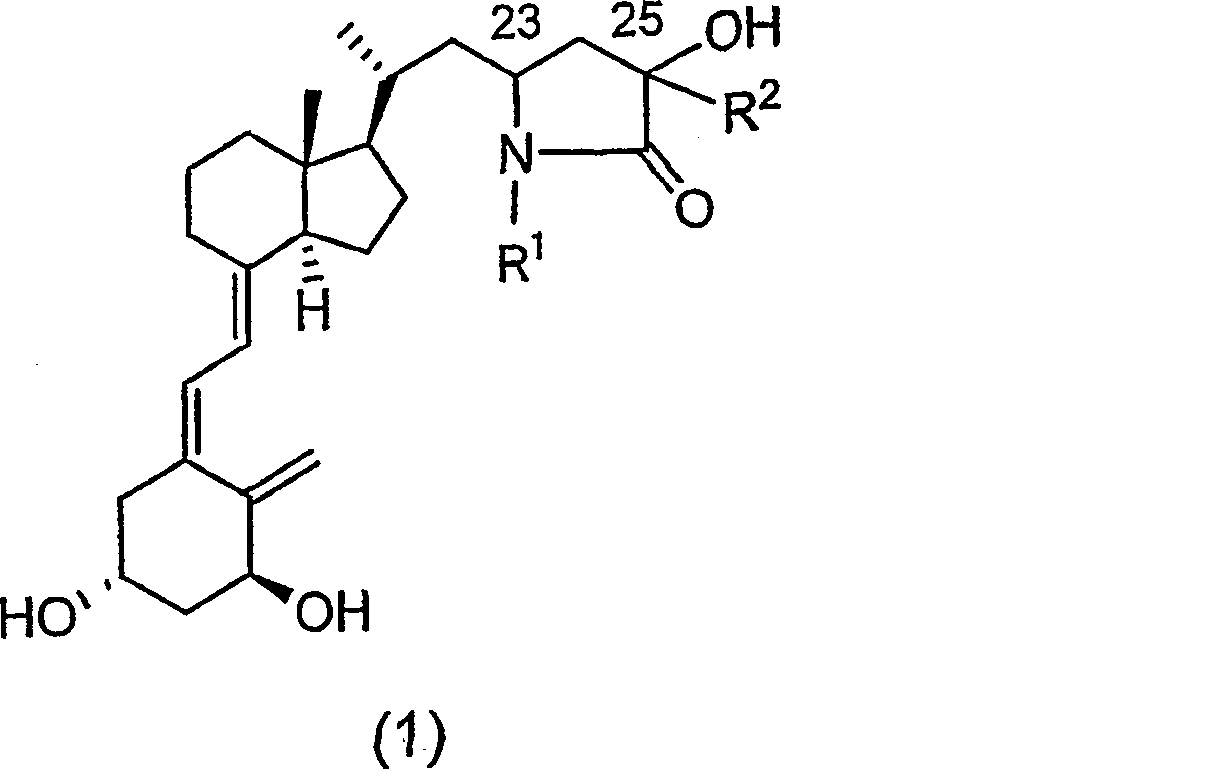
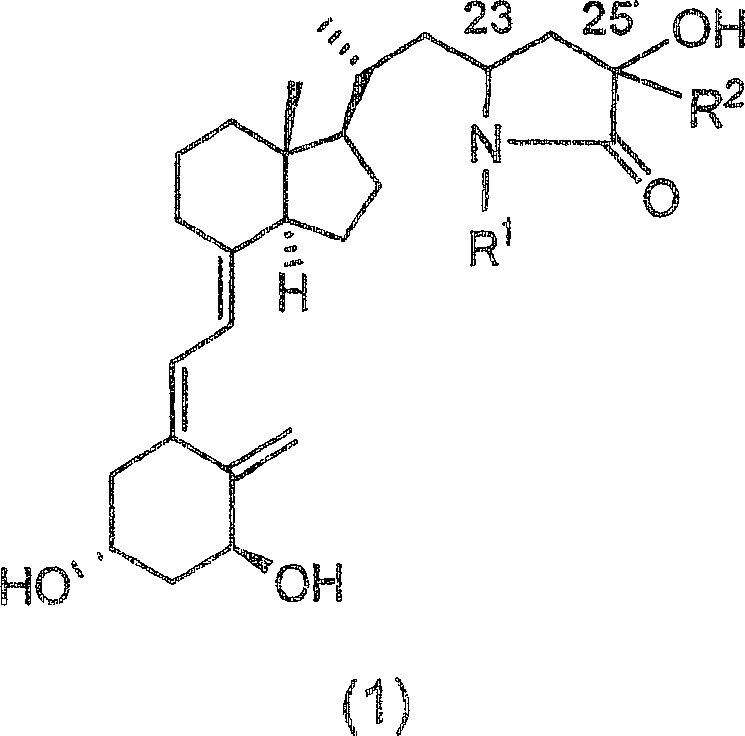
Vitamin d3 lactam derivative
A technology of derivatives and vitamins, applied in the field of vitamin D3 lactam derivatives or their pharmaceutically acceptable solvates, can solve problems such as accumulation and lack of knowledge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040] Below, the present invention will be described in further detail through examples, but the present invention is not limited thereto. The compound number in each example corresponds to the compound number shown in the above table, or the compound number in the above reaction scheme 1 or 2.
reference example 1
[0042] Preparation of 2-phenylethylhydroxylamine
[0043]
[0044] In a nitrogen atmosphere, at 0 °C to BH 3 - THF solution in THF 1.1 mL (1.0 M, 1.1 mmol) was added dropwise with a THF solution (2.2 mL) of 160.0 mg trans-β-nitrostyrene (1.1 mmol). Add 3.3 mg of NaBH 4 (0.087mmol), stirred at room temperature for 20 minutes. Add 5 mL of water at 0°C, add 2M aqueous HCl to make the solution acidic, and stir at 65°C for 4 hours. After extraction with ethyl acetate, a 15% aqueous NaOH solution and NaCl were added to the aqueous layer, followed by further extraction with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered, and the solvent was distilled off. The residue was purified by flash column chromatography (chloroform:methanol=1:0→10:1) to obtain the desired product (62.7 mg, 43%) as white crystals.
[0045] 1 H NMR (CDCl 3 ) δ: 7.47 (brs, 1H), 7.31-7.20 (m, 5H), 3.26 (t, J=7.3Hz, 2H), 2.97 (t, J=7.3Hz, 2H).
reference example 2
[0047] Preparation of 3-phenylpropyl hydroxylamine
[0048]
[0049] (1) Add 166.7 mg of hydroxylamine hydrochloride (2.40 mmol) and 0.7 mL of Et 3 N (5.05 mmol), stirred at room temperature for 2 hours. Add saturated aqueous sodium bicarbonate solution at 0°C, and extract with dichloromethane. After drying over anhydrous magnesium sulfate, it was filtered and the solvent was distilled off. The residue was purified by flash column chromatography (hexane:ethyl acetate=5:1) to obtain white crystalline Compound A (190.0 mg, 100%).
[0050] 1 H NMR (CDCl 3 )δ: 7.47(t, J=6.0Hz, 1H), 7.32-7.19(m, 5H), 6.80(brs, 1H), 2.83(dd, J=7.3, 15.0Hz, 2H), 2.74(m, 1H ), 2.54(m, 1H).
[0051] (2) Add 13.8 mg of NaBH to 46.8 mg of Compound A (0.31 mmol) in methanol solution 2.0 mL at room temperature 3 CN (0.22 mmol). 2M HCl-MeOH solution was added to maintain pH = 3, and stirred at room temperature for 2 hours. After adding 15% NaOH aqueous solution to make it basic, sodium chlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com