Cannabidiol combination compositions
A technology of cannabidiol and composition, which is applied in the field of cannabidiol joint composition, can solve problems such as no improvement, no effective, pain relief or no joint damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1 - Permeation
[0092] Three different oral formulations of water soluble CBD were tested, F#01 (20%), L#02 (2.5% CBD) and I#03 (20% CBD), and were administered to rats at 15 mg / Kg.
[0093] Three formulations were tested in combination with 500 mg / Kg glucosamine (Gln).
[0094] The total application amount was 10 mL / Kg (CBD+Gln).
[0095] Tests were performed on Sprague Dawley male rats (n=3 / group).
[0096] A single dose of PO containing CBD and GIn was given at t=0.
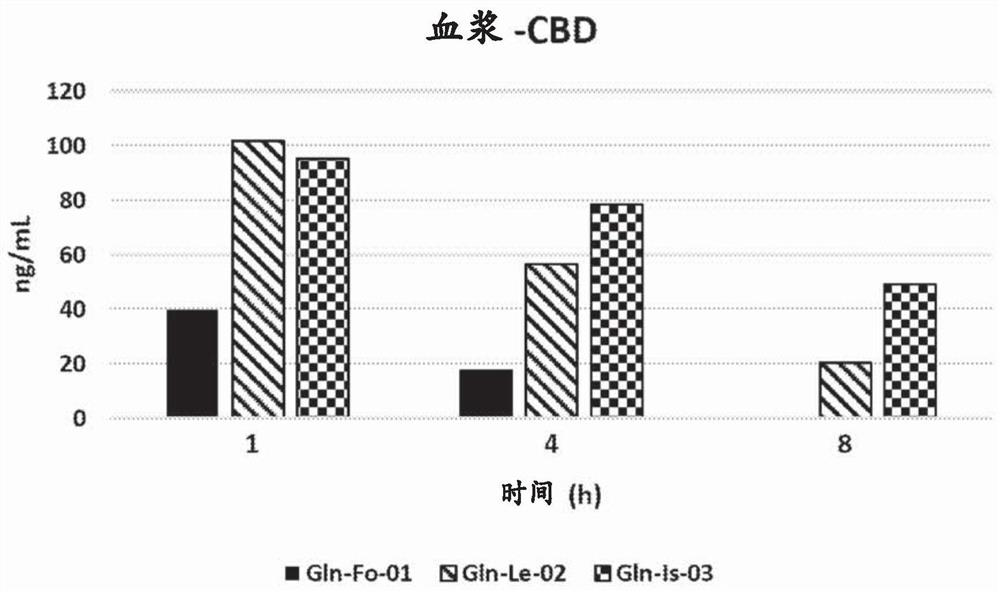
[0097] CBD and Gln plasma concentrations were measured 1, 4 and 8 hours after a single administration.
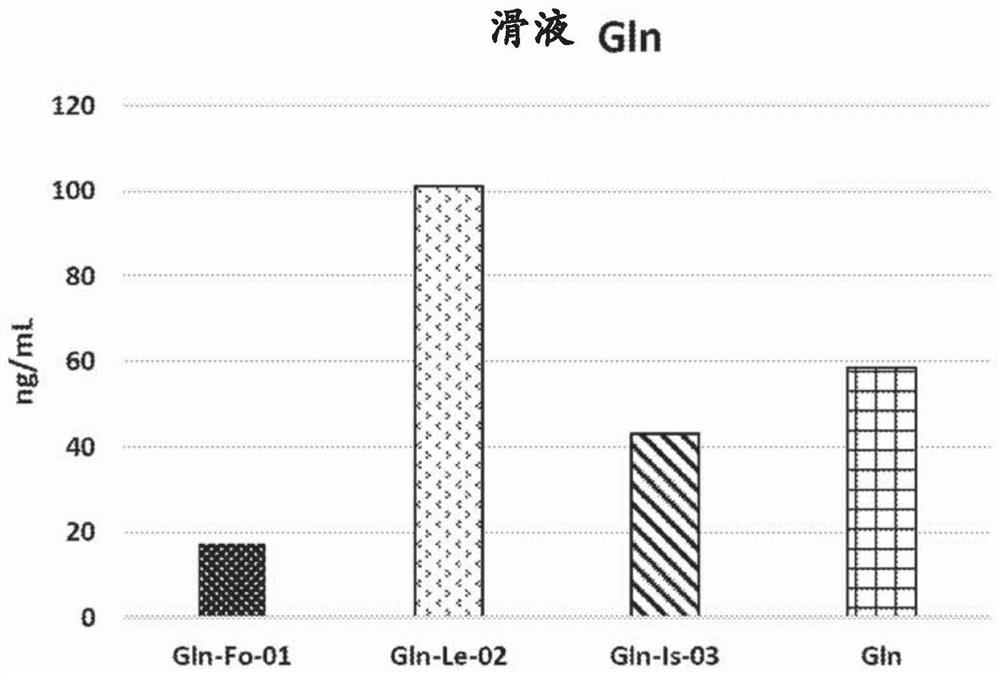
[0098] Eight hours after oral administration, the concentration of Gln in the synovial fluid was measured.
[0099] result:
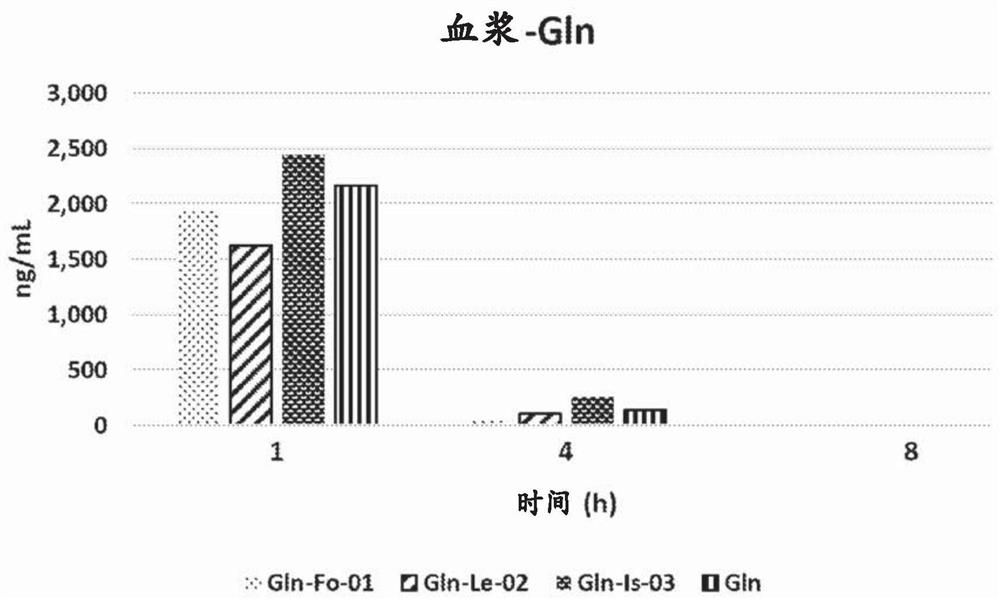
[0100] Such as figure 1 As shown, no significant difference in Gln plasma concentration was found among the groups.
[0101] One hour after administration, the average Gln plasma concentration was 2.039 ng / mL, which dropped to 134 ng / mL (93.4% lower than the ba...
Embodiment 2
[0108] Example 2 - Dog Treats
[0109] Two compositions comprising CBD and Gln were formulated as dog treats.
[0110] Composition A for small dogs contained 2.5mg CBD and 400mg Gln in a 6g treat.
[0111] Composition A for medium-sized dogs contained 4.5mg CBD and 700mg Gln in a 10g treat.
[0112] Composition A for large dogs contained 7.5mg CBD and 1.2g Gln in a 12g treat.
[0113] Composition B contained 10 mg CBD and 1.5 g Gln.
[0114] Each composition was formulated into a dog treat using soft dog treats and flavorings. Figure 5 It is a photograph of dog snacks.
[0115] Treats were administered to 5 healthy dogs.
[0116] Dogs readily snacked, indicating that the canine treat preparation had a desirable taste, while the bad taste of CBD was masked.
Embodiment 3
[0117] Example 3 - Ratio
[0118] The trial included approximately four animal groups (3 for synovial fluid and 1 for plasma) for four different CBD:Gln ratios (1:5, 1:10, 1:20 and 1:30) , which included two CBD formulations and a control (Gln only). Each of these 48 groups housed 8 rats for a total of 384 animals (128 in plasma vs. 256 in synovial fluid).
[0119] The total dose is 10 mL / Kg.
[0120] Tests were performed on Sprague Dawley male rats (n=3 / group).
[0121] A single dose of PO was given at t=0.
[0122] CBD and Gln plasma concentrations were checked 1, 4 and 8 hours after a single administration.
[0123] Examine synovial fluid 8 hours after oral administration.
[0124] The goal is to optimize the CBD:Gln ratio to achieve maximum Gln concentration in synovial fluid while maintaining acceptable and applicable oral doses of CBD and Gln.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com