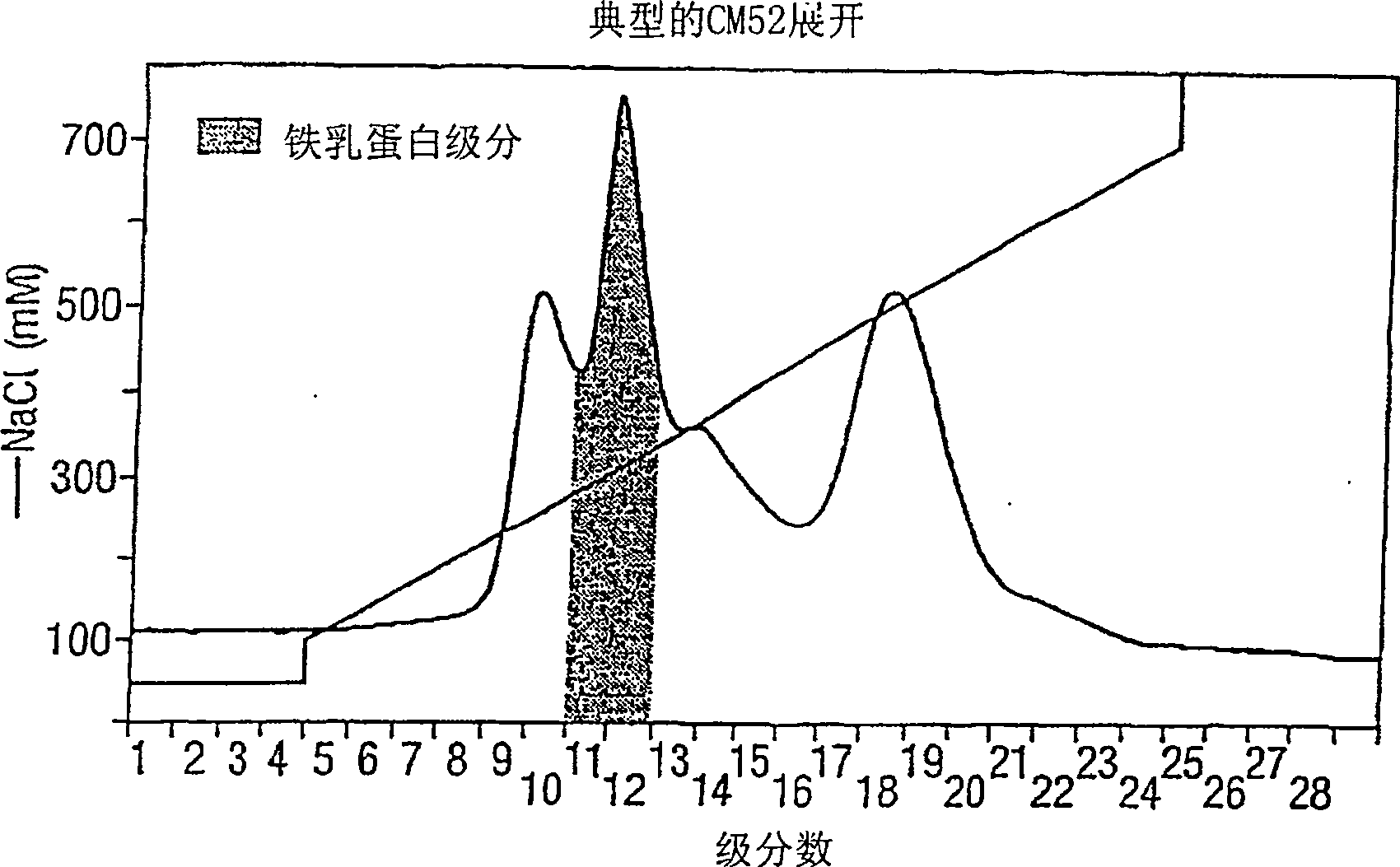
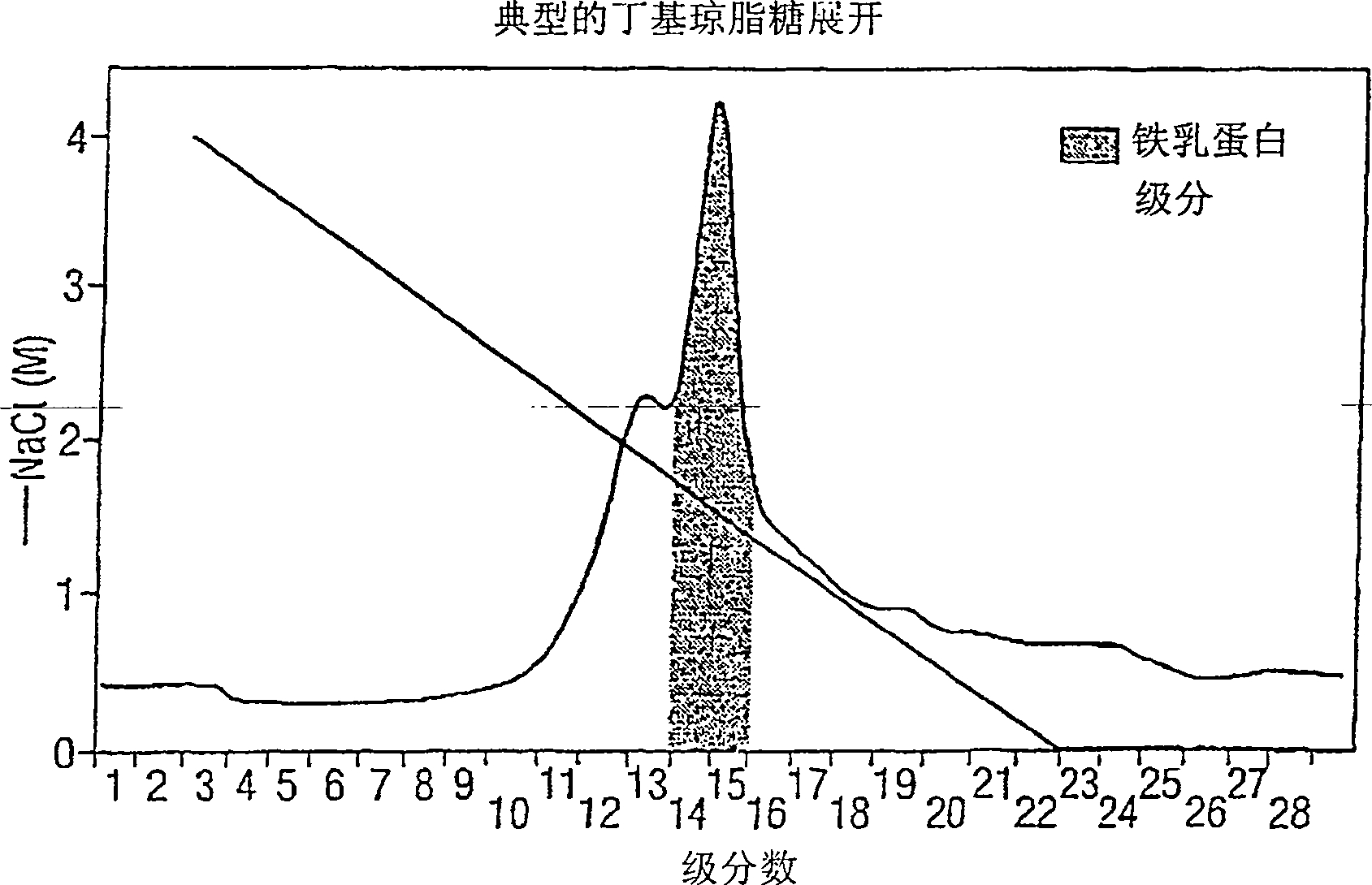
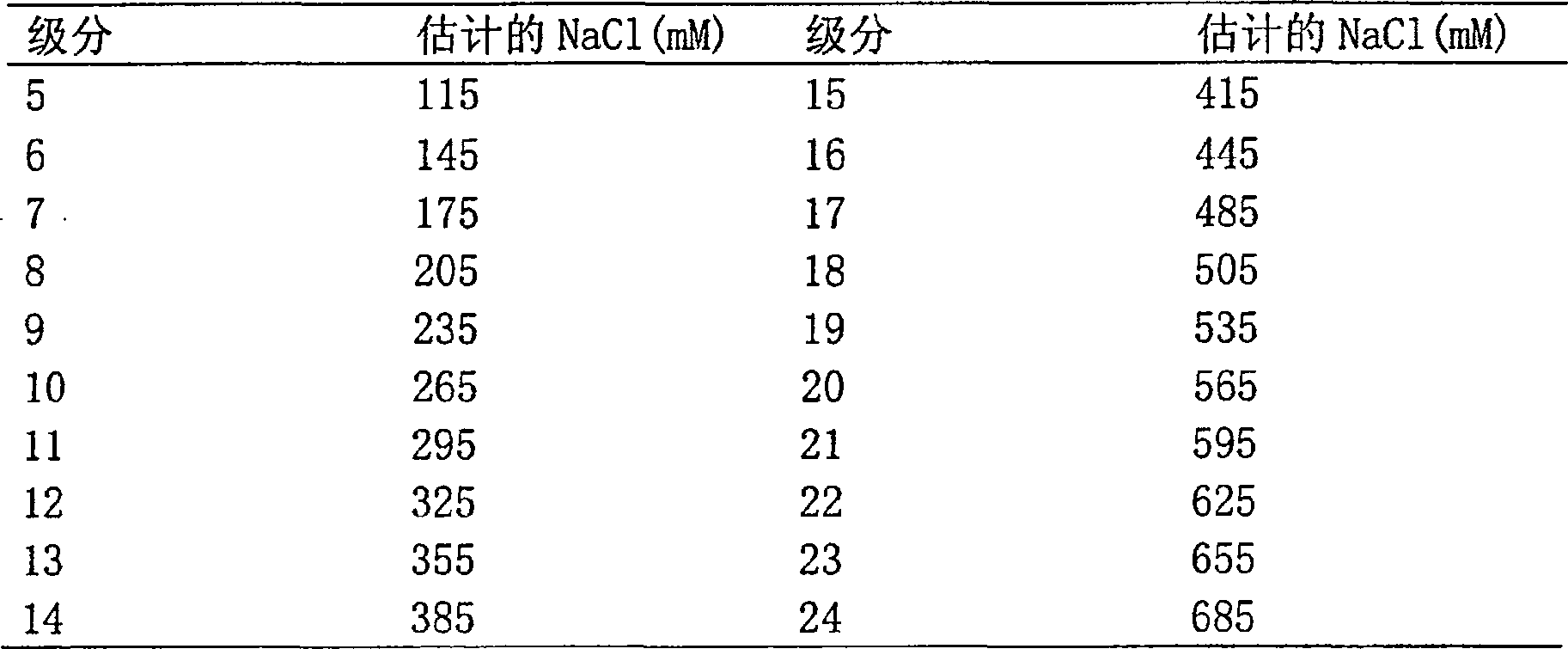
Peptide composition
A drug and amino acid technology, applied in the field of peptide application, can solve problems such as low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0262] Example 1 - Preparation of standardized natural products from cheese whey
[0263] This step includes methods for collecting, preparing and storing standardized natural products (SNPs) from cheese whey. This step is often used to prepare SNPs on a small scale, such as for research or improvement purposes. However, according to the prior art, this method can be scaled up for commercial production as needed.
[0264] Collection and storage of cheese whey
[0265] Approximately 401 fresh pure cheese whey was obtained from DewLay Cheese Factory (Garstang, Lancashire). The whey is collected in clean containers and sent immediately to the Pepsyn Central Manufacturing Plant (Liverpool).
[0266] The whey can be refrigerated for later processing, or it can be frozen at -20°C in a shallow container at 21 until use.
[0267] Thawing of Cheese Whey
[0268] Place 21 whey cubes in a plastic bag and submerge in hot running water to thaw the whey. Thawing is completed within 10...
Embodiment 2
[0282] Example 2 - Demonstration that SNPs increase collagen synthesis in fibroblasts
[0283] Rama27 rat cells were grown to confluence and their rate of collagen synthesis was measured by the method of M.J. Warburton, S.A. Ferns and P.S. Rudland, Experimental Cell Research, 137, 373-380 (1982). Collagen synthesis rates calculated by incorporating [3H]proline into hydroxyproline are listed in Table 2 below:
[0284] Table 2. Rate of Collagen Synthesis
[0285]
[0286] Addition of 0.6 mg / ml of SNP increased collagen secretion approximately 12-fold—from 54 cpm to 663 cpm. This also approximately tripled the ratio of secreted synthetic collagen to collagen contained in the cells—from 54:53 (1:1) to 663:321 (2:1).
Embodiment 3
[0287] Example 3 - Demonstration of the effect of SNPs on keratinocyte growth
[0288] Human keratinocytes (HatKat) were grown in Keratinocyte Growth Medium (TCS Cellworks Ltd.) until 20% confluent. Then, 0.5% fetal calf serum (FCS) was added to the same medium, and the keratinocytes were grown for 3 days. The counter counts the cells. The resulting cell numbers are listed in Table 3 below.
[0289] Table 3. Cell Numbers
[0290]
[0291] This indicated that the presence of the SNP increased keratinocyte growth approximately 3-fold—from 23,777-68,719. In contrast, the growth obtained with 10 ng / ml EGF was relatively moderate.
[0292] These results clearly demonstrate the collagen-producing and growth-promoting activity of the peptides used in the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com