CHO cell strain for highly effective expressing rhBMP2 and establishing method thereof
A high-efficiency expression, cell line technology, applied in the field of genetic engineering pharmaceuticals, can solve the problem that the recombinant CHO cell line has not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
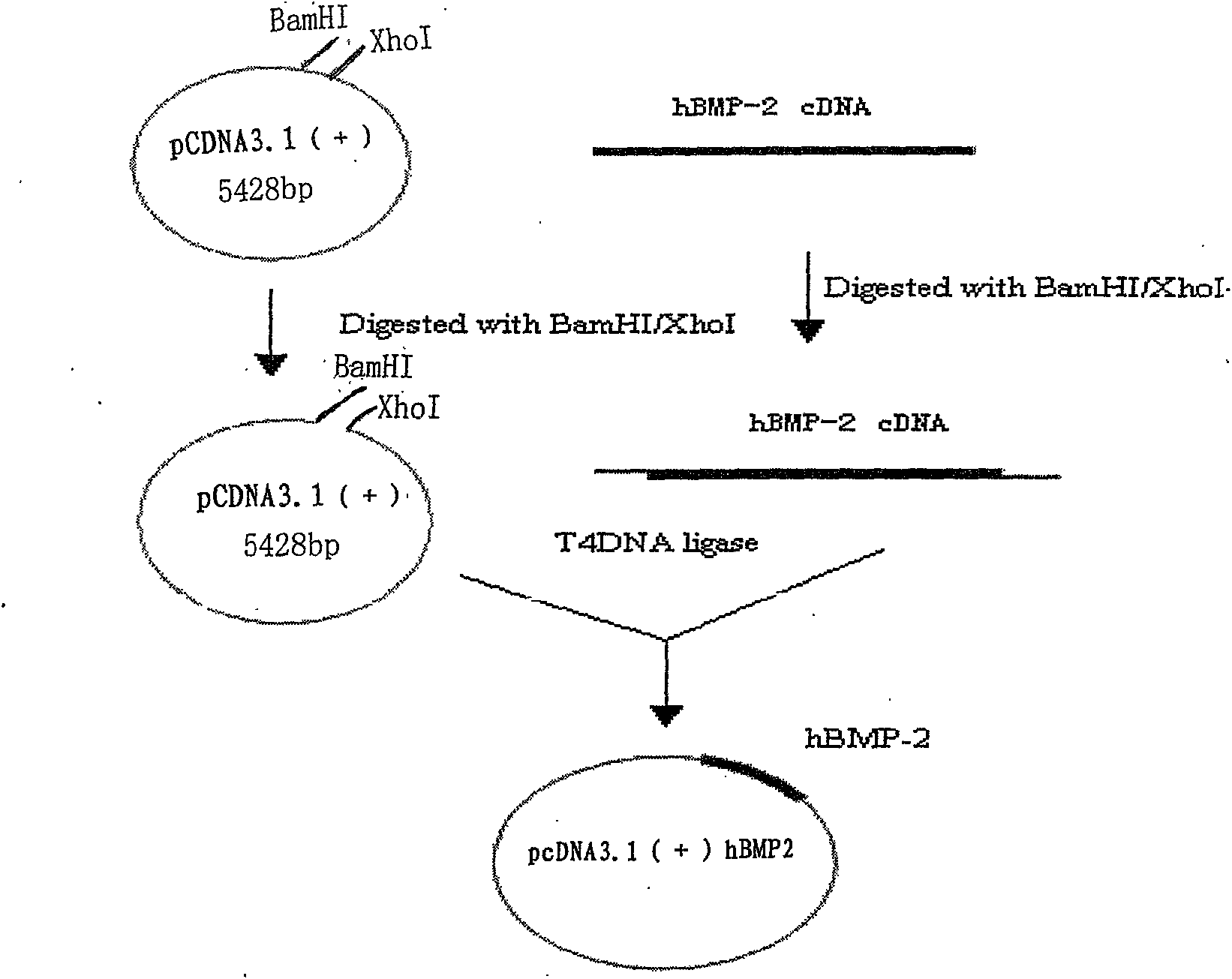
[0065] Example 1: Construction of pCDNA3.1(+)-BMP2
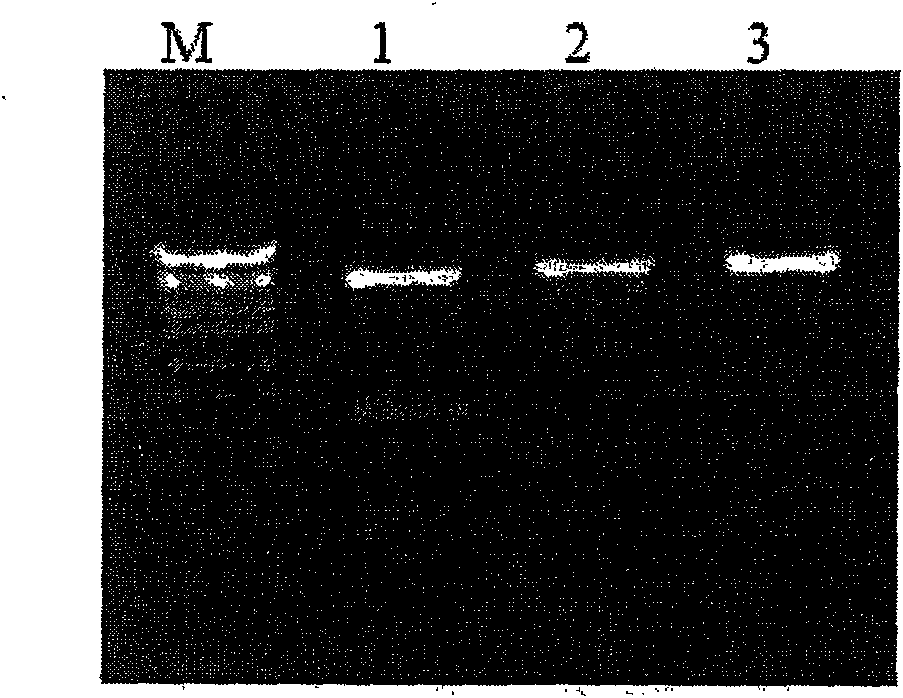
[0066] According to the gene sequence of human bone morphogenetic protein 2 (BMP2, SEQ ID NO. 1), two pairs of specific primers (B2F, B2R) were designed and synthesized. Using B2F and B2R as primers, polymerase chain reaction was used to amplify a fragment with a size of about 1200bp ( figure 2 A). This fragment was cloned into a eukaryotic expression vector to obtain pCDNA3.1(+)-BMP2, which was digested ( figure 2 B). Sequencing confirmed that it was consistent with the expected target sequence.
[0067] B2F: 5’-gctggatccaccatggtggccgggacccgctgtc-3’
[0068] B2R: 5’-gcgtctcgagctagcgacacccacaaccctccac-3’
Embodiment 2
[0069] Example 2: Establishment of an engineered cell line stably and efficiently expressing recombinant human BMP2
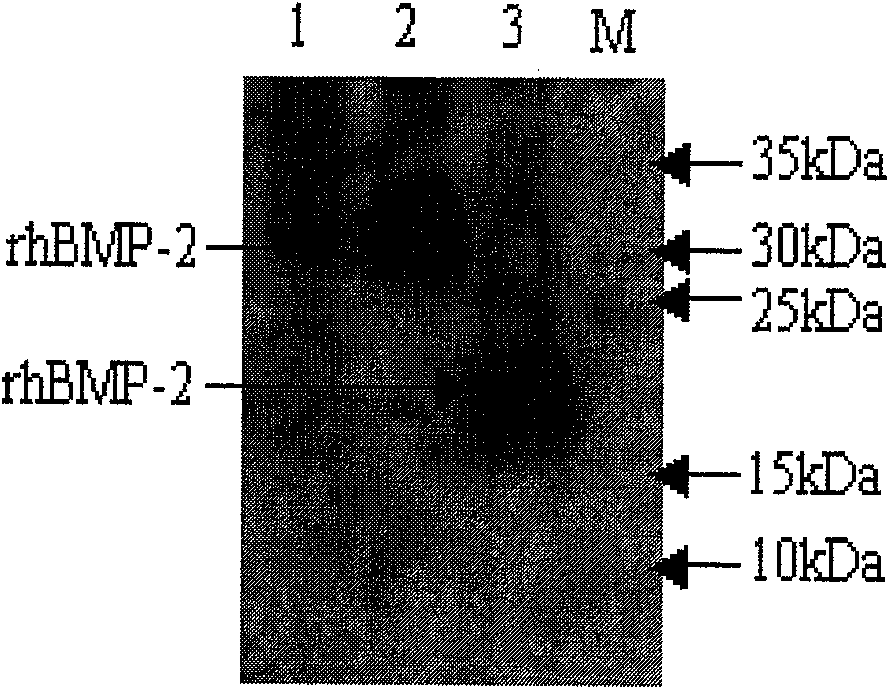
[0070] Dihydrofolate reductase-deficient Chinese hamster ovary cells (CHO-dhfr-) with a complete medium supplemented with glycine, hypoxanthine, thymine 10% FBS IMEDM (Hyclone), at 37℃, 5% CO2, 10cm culture plate Culture the cells to about 80% confluence. Use cationic liposome Lipofectine 2000 (Invitrogen) to transfect pCDNA3.1(+)-BMP2 and pSV2-DHFR at a ratio of 10:1 according to the operation manual, and then use the selection of IMDM containing 700ug / ml G418 and 10% FCS The medium was used to screen neo and dhfr positive clones. The positive clones obtained are 1×10 6 / 10ml cells were inoculated in a 10cm cell culture dish, and the cells were gradually cultured in IMDM medium containing 100nM and 1uM methotrexate (MTX) and 10% FBS to amplify the target gene, and finally obtain a medium containing 1uM MTX Recombinant cells that grow normally. Extract the cell cultu...
Embodiment 3
[0071] Example 3: In vitro bone formation activity analysis of recombinant human BMP2
[0072] When the myoblast cell line C2C12 was cultured in DMEM containing 10% FBS to 70-80% confluence, washed with PBS, trypsinized, and counted to 2x10 4 Each cell / well was seeded in a 48-well cell culture plate and incubated in a 37°C, 5% CO2 incubator. After 24 hours, the medium was aspirated, washed twice with PBS, and conditioned medium containing 5%, 10%, and 20% rCHO(BMP2) 24h cell culture medium was added to stimulate the culture of C2C12. After 5 days, count with CCK-8 kit The number of cells per well, and then the colorimetric method to measure the alkaline phosphatase activity ( Figure 4 ), the highest expression level can reach 7.83μg / 24h / 10 6 cell.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com