Medicine composition for treating kidney-yang deficiency and sexual function decrement, and its prepairng method
A technology of hypofunction and composition, which is applied in the field of pharmaceutical composition and preparation technology for treating kidney yang deficiency and sexual hypofunction, which can solve the problems of liver and kidney function damage, drug dependence, and side effects of the body, and improve the body Immunity status and various organ system functions, enhanced libido, and energetic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0025] Experimental Example 1 The androgen-like effect of the capsule of the pharmaceutical composition of the present invention
[0026] Fifty healthy male SD juvenile rats weighing 35-40g were selected and randomly divided into 5 groups with 10 rats in each group. The first group was blank control, 0.5% sodium carboxymethylcellulose solution was orally administered, the second group was testosterone propionate positive group, intramuscular injection every other day, the dose was 25mg / kg, groups 3, 4 and 5 were the drug combination of the present invention Drug capsules, the doses are 200, 600 and 1800 mg / kg respectively. 1ml / 100g WT was orally administered daily for 1 month. Twenty-four hours after the last administration, the animals were weighed and sacrificed. The prostate and seminal vesicles were taken out, accurately weighed, and finally converted into 100 g body weight of the prostate and seminal vesicles.
[0027] Table 1 The androgen-like effects of the pharmaceutical c...
experiment example 2
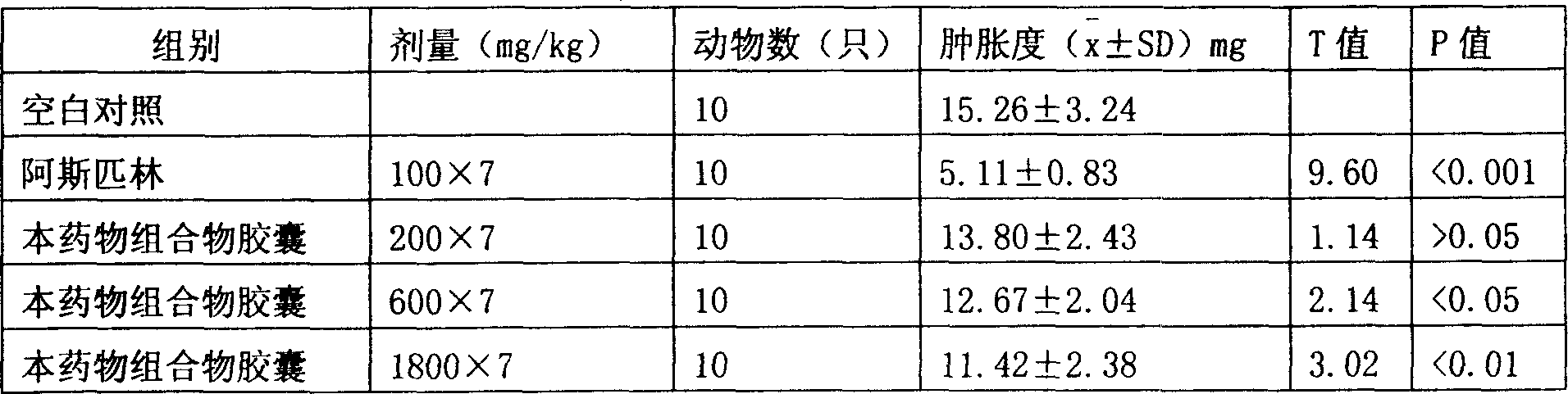
[0030] Experimental example 2 The effect of the pharmaceutical composition capsule of the present invention on the ear swelling caused by xylene
[0031] Fifty healthy male Kunming mice weighing 25-30g were selected. Randomly divided into 5 groups with 10 animals in each group. Group 1 is a blank control group, 0.5% sodium carboxymethyl cellulose solution is orally administered, group 2 is aspirin-positive group, oral dose is 100 mg / kg, group 3, 4 and 5 are capsules of the pharmaceutical composition of the present invention , The doses are 200, 600 and 1800 mg / kg respectively. Take 0.5ml / 20g orally every day for 7 consecutive days. Thirty minutes after the last administration, 0.05 ml of xylene was dropped into the left ear of the mouse. After 30 minutes, cut off the two ears, punched the two ears with a 0.7cm hole punch, and weighed them on an analytical balance. The difference in the weight of the two ears was used as an indicator of the degree of swelling.
[0032] Table 2 The ...
experiment example 3
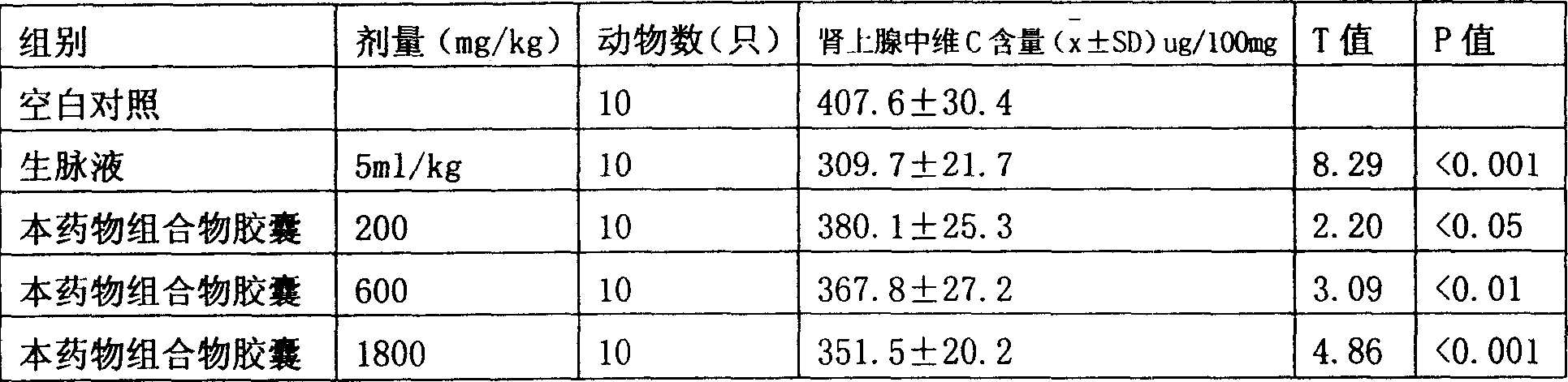
[0034] Experimental Example 3 The effect of the pharmaceutical composition capsule of the present invention on the content of vitamin C in the adrenal glands of rats
[0035] Fifty healthy male SD rats weighing 130~150g were selected and randomly divided into 5 groups with 10 rats in each group. Group 1 was blank control, 0.5% sodium carboxymethyl cellulose solution was orally administered, group 2 was positive intravenous injection of Shengmai liquid, the dose was 5ml / kg, group 3, 4 and 5 were capsules of the pharmaceutical composition of the present invention. For 200, 600, and 1800 mg / kg, 1ml / 100g WT was orally administered daily for 7 consecutive days. The animals were sacrificed 1 hour after the last administration, and the bilateral adrenal glands were quickly dissected, and the surrounding tissues around the adrenal glands were carefully separated and weighed with a torsion balance and put into a tissue homogenizer containing 1.5ml of 5% trichloroacetic acid. Homogenize, ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com