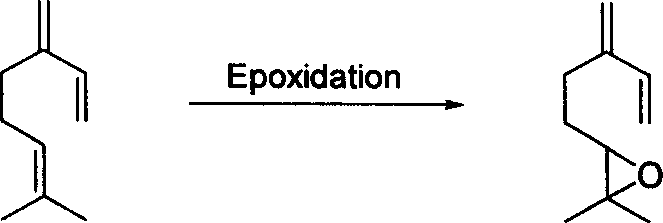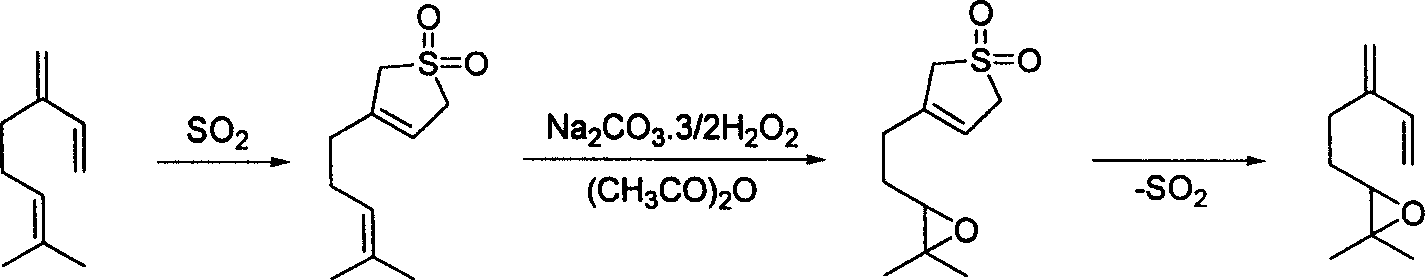Method for preparing epoxy myrcene
A technology of epoxy myrcene and myrcene, which is applied in the field of preparation of epoxy myrcene, can solve problems such as difficult separation and purification of products, and achieve the effects of safe operation, low cost and excellent product performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of myrcene sulfone: add 200g myrcene (industrial product, content about 75%, 1.1mol), 2g (9mmol) 2,6-di-tert-butyl-4-methylphenol and 200g (3.1mol) in the autoclave Sulphur dioxide. Heating and stirring, the temperature is controlled at 65 ~ 75 ℃. After the pressure in the reactor was stable and no longer decreased, the reaction was continued for 2 hours. After the reaction is completed, the remaining sulfur dioxide in the reactor is vented, and the vented gas is absorbed by alkaline water.
[0028] The obtained reactant was subjected to vacuum distillation, and the fractions before 85°C / 5mmHg were impurities that did not participate in the reaction in the raw materials, and they were removed as much as possible. The residue is the crude product of myrcyl sulfone with a purity of about 90-95%. According to the actual content of myrcene in the raw material, the yield is close to 95%. The crude product was used in the next epoxidation reaction without furt...
Embodiment 2
[0033] Add 100g (0.5mol) myrcyl sulfone prepared in Example 1, 500mL xylene and 120g sodium percarbonate into the three-necked flask, slowly add 100mL acetic anhydride under vigorous stirring, the reaction exotherm is obvious, control the dropping rate of acetic anhydride , the reaction temperature was maintained at 40-60 °C. After the addition, the reaction was carried out at room temperature overnight, and the inorganic solid was removed. The filtrate is removed by conventional methods to remove residual peroxy compounds, and the solvent is recovered under reduced pressure to obtain a crude product of myrcene sulfone epoxidation product. The crude product was decomposed while heating, and distilled under reduced pressure, and the fractions at 75-80°C / 10mmHg were collected until the decomposition was complete. 56 g of colorless liquid were obtained in 78% yield.
[0034] Within the scope of the various components and conditions described in the method of the present inventi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com