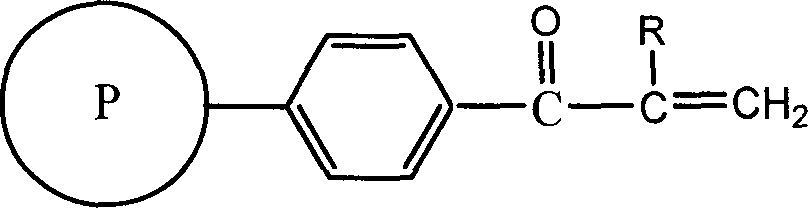
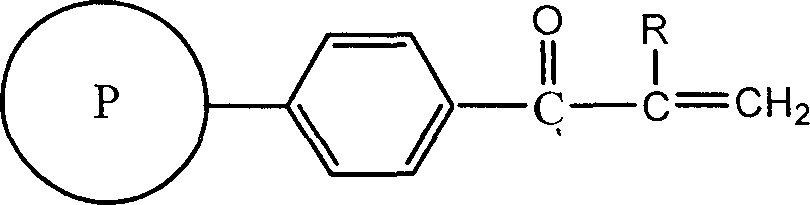
Polystyrene resin of containing double bond, and preparation method
A polystyrene resin and resin technology, applied in the field of functional polymers, can solve the problems of low double bond content and low cross-linking degree, and achieve the effects of simple reaction steps, high double bond content and high degree of substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take 1g of polystyrene (cross-linking degree: 80% DVB, particle size: 5μm) into a three-neck reaction flask, add anhydrous dichloromethane to swell, stir to make it evenly dispersed, add 1ml of acryloyl chloride dropwise, blow in nitrogen, and stir 1.95 g of aluminum trichloride was added under the pressure, and the reaction was carried out at normal temperature and pressure for 4 hours. That is, a double bond-containing polystyrene resin having a double bond content of 0.93 mmol / g was obtained.
Embodiment 2
[0020] Take 1g of polystyrene (cross-linking degree: 7% DVB, particle size: 20μm) into a three-necked reaction flask, add anhydrous dichloroethane to swell, stir to make it evenly dispersed, add dropwise 1ml of acryloyl chloride, and blow in nitrogen, Add 0.64 g of aluminum trichloride under stirring, and react at room temperature and pressure for 1 hour. That is, a double bond-containing polystyrene styrene resin having a double bond content of 2.50 mmol / g was obtained.
Embodiment 3
[0022] Take 1g of polystyrene (cross-linking degree: 55% DVB, particle size: 20μm) into a three-necked reaction flask, add anhydrous nitrobenzene to swell, stir to make it evenly dispersed, add 1ml of acryloyl bromide dropwise, and blow in nitrogen. Add 0.3ml SnCl with stirring 4 , React at room temperature and pressure for 15 minutes. That is, a double bond-containing polystyrene resin having a double bond content of 2.17 mmol / g was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com