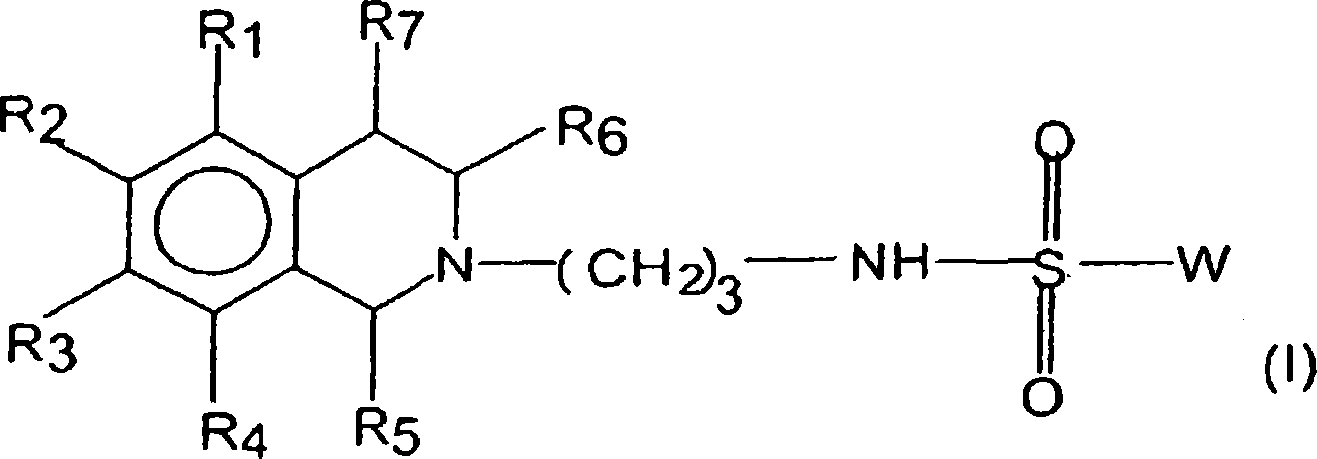
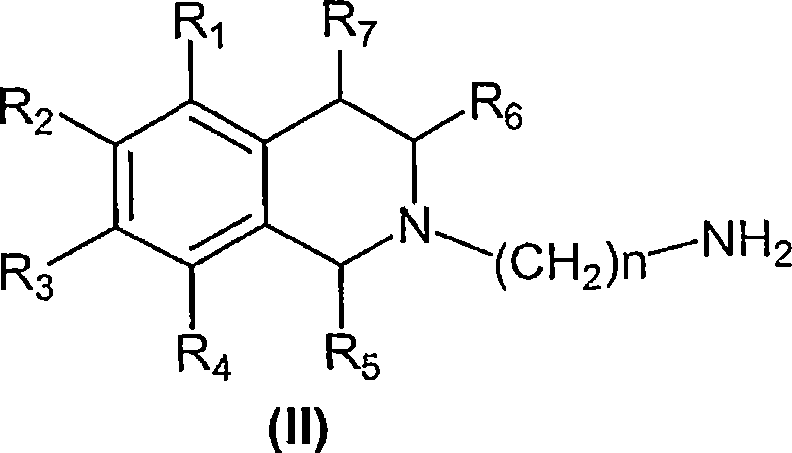
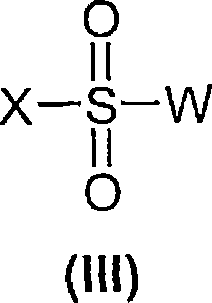5-HT7 receptor antagonists
A technology of isomers and solvates, applied in the field of 5-HT7 receptor antagonists, can solve the problems of unreported activity and low sequence homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0120] Synthesis of compounds of general formula (II)
[0121]
[0122] a) 2-[3-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-propyl]-isoindole-1,3-dione
[0123] At room temperature, 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (6.89 g, 0.030 mol ), N-(3-bromopropyl)phthalimide (8.04g, 0.030mol), potassium carbonate (16.50g, 0.120mol) mixture was stirred overnight. The mixture was concentrated in vacuo and the residue was dissolved in water (120 mL) and extracted with ethyl acetate (3 x 30 mL), washed with water, the organic layer was dried and evaporated to give the product (10.85 g, 93% yield), which The product was used without further purification.
[0124] b) 3-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-propylamine dihydrochloride
[0125] 2-[3-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-propyl]-isoindole-1,3-dione (10.46 g, 0.0275mol) and hydrazine hydrate (8.6mL, 0.275mol) in ethanol (250mL) were refluxed for 1 hour. The reaction mixtur...
Embodiment B
[0130] Compounds of general formula (I) are prepared by coupling compounds of general formula (II) with compounds of general formula (III) according to conventional methods of organic chemistry known to those skilled in the art.
[0131] 5-Chloro-N-[3-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-propyl]-2,4-difluoro-benzenesulfonate Amide (Compound 48)
[0132]
[0133] 3-Chloro-4,6-difluorobenzenesulfonyl chloride (259 mg, 1.05 mmol) was added to 3-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl )-Propylamine dihydrochloride (323mg, 1mmol), N, N-diisopropylethylamine (517mg, 4mmol) CH 2 Cl 2 (15 mL) solution and stirred overnight at room temperature. The resulting solution was washed with water (3×20 mL), washed with Na 2 SO 4 The organic layer was dried and evaporated to dryness. The free base was dissolved in 2-propanol (5 mL). The product was crystallized in 2-propanol (5 mL) and collected by filtration and dried in vacuo to give a white solid (401 mg, 87%).
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com