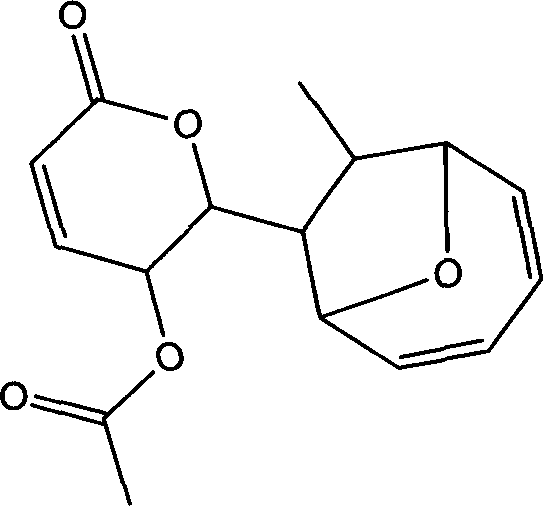
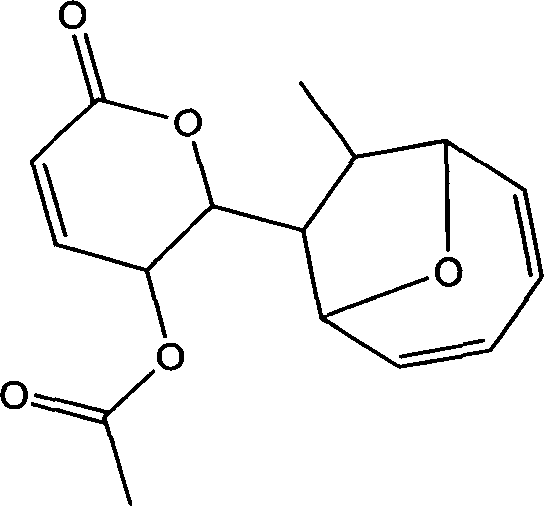
Use of compound metabolized by diaporthe phaseolorum fungus
A compound and fungal technology, applied in the field of compounds produced by fungal metabolism, can solve problems such as unreported anti-tumor physiological activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Take the strains on the slant and inoculate them in semi-seawater PD and cultivate them until fermentation. The fermentation broth is filtered through gauze to remove the bacteria, and the supernatant is collected. The collected supernatant is divided into 3L conical flasks (1L / bottle), and the same The volume of ethyl acetate was stirred and extracted for 6 hours; the organic phase was collected and concentrated under reduced pressure at 50°C with a rotary evaporator to a paste. Get the silica gel (80 order) that quality is 8 times of loading sample amount, use dry method to pack column, column internal diameter is selected as 6cm, and column length is 8cm, and the sedimentation of silica gel is sucked with vacuum pump to no longer change; The sample after concentration is used After mixing the sample with silica gel (60 mesh), it is compacted and spread flat on the cylinder surface, and then the surface of the sample is covered with sea sand with a thickness of 1 cm. ...
Embodiment 2
[0028] Its preparation process is similar to Example 1, the difference is that the collected supernatant is divided into 3L Erlenmeyer flasks (1L / bottle), and the addition of ethyl acetate with a volume of 0.8 times the fermentation broth is stirred and extracted for 10h; the organic phase is collected, Concentrate under reduced pressure at 45°C with a rotary evaporator to a paste. Take 60-mesh silica gel whose mass is 6 times of the sample volume, and pack the column by dry method. The concentrated sample was mixed with 100-mesh silica gel, compacted and spread on the cylinder surface, and then covered with sea sand with a thickness of 1 cm on the sample surface. The temperature for concentration under reduced pressure was 55°C. When performing further silica gel column separation, take 60 mesh silica gel whose mass is 8 times of the loading amount.
[0029] The antitumor activity of the compound was determined by cytotoxic MTT assay, and it was found that its IC on human o...
Embodiment 3
[0031] Its preparation process is similar to Example 1, and its difference is that the collected supernatant is divided into 3L Erlenmeyer flasks (1L / bottle), adding ethyl acetate whose volume is 1.2 times of the fermentation broth and stirring and extracting for 15h; collecting the organic phase, Concentrate under reduced pressure at 55°C with a rotary evaporator to a paste. Take 100-mesh silica gel whose mass is 10 times of the sample volume, and pack the column by dry method. The concentrated sample was mixed with 80-mesh silica gel and spread flat on the cylinder surface, and then covered with sea sand with a thickness of 1 cm on the sample surface. The temperature for concentration under reduced pressure was 45°C. When performing further silica gel column separation, take 100 mesh silica gel whose mass is 6 times of the loading amount.
[0032] The antitumor activity of the compound was determined by cytotoxic MTT assay, and it was found that its IC on human oral dermoi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com