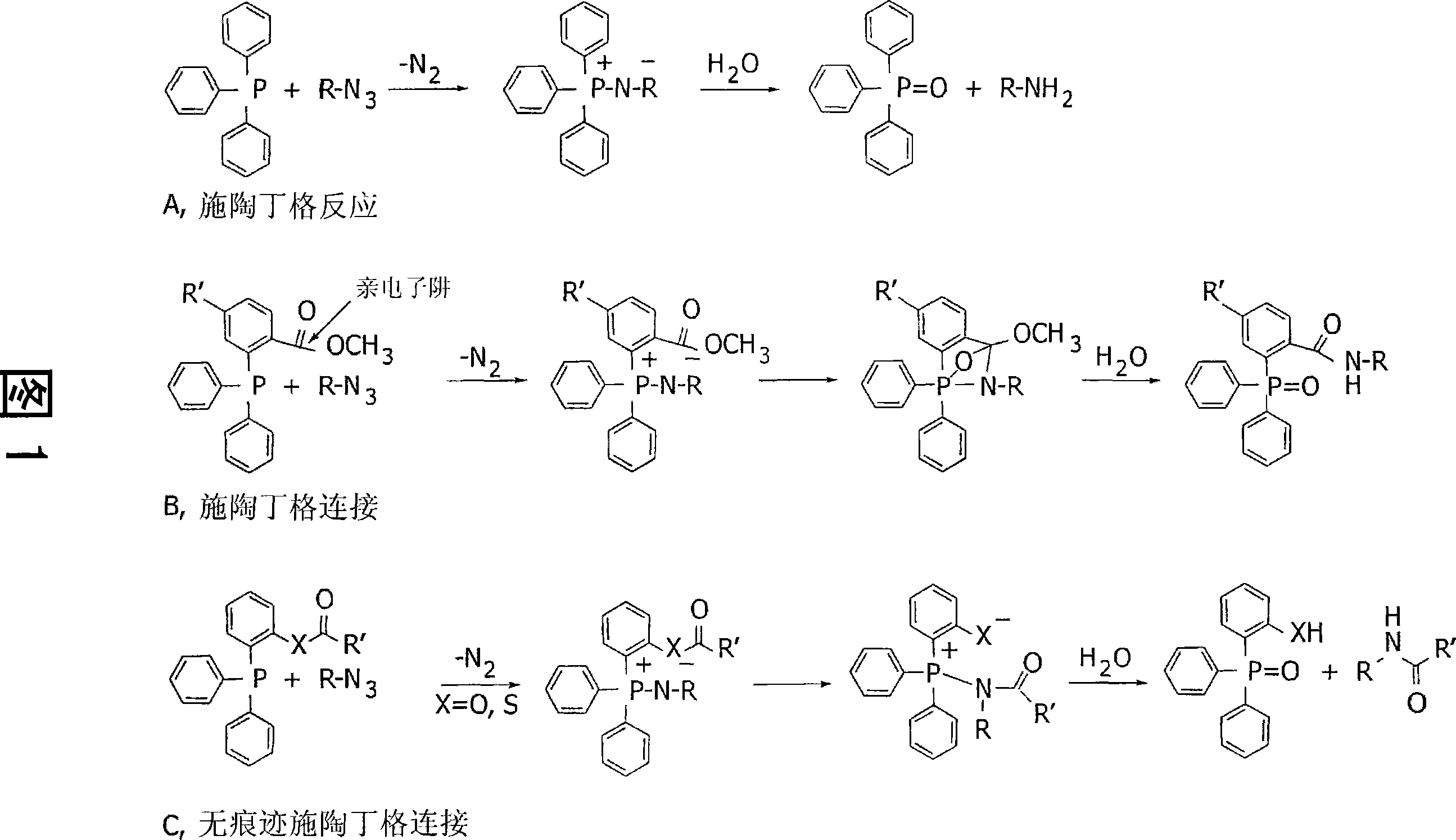
Compounds, kits and methods for use in medical imaging
A technology of kits and imaging agents, applied in the field of new compounds for medical imaging and treatment, which can solve problems such as blurring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Pretargeted Imaging of Tumors Using Azide-Glucose
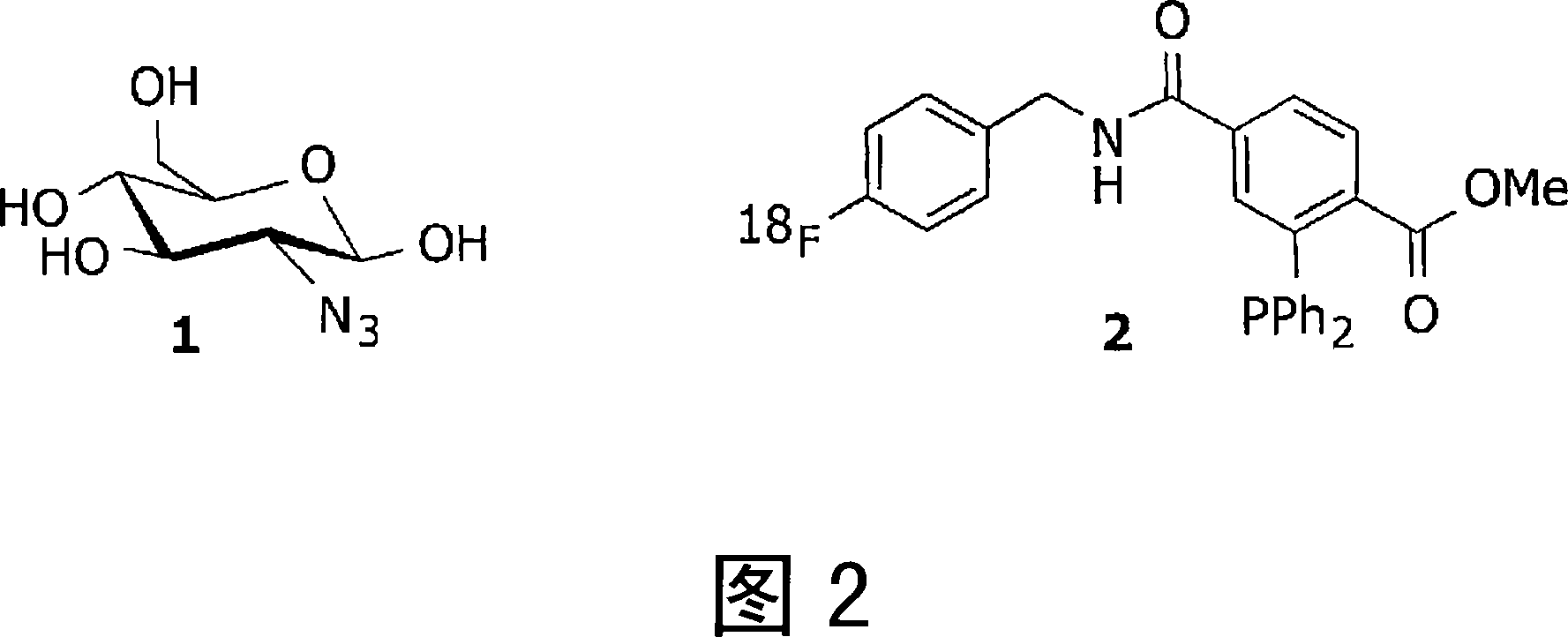
[0067] Refer to Figure 2.
[0068] Systemic administration of azide-glucose probe 1. Following optimal accumulation in tissues with high glucose uptake (such as tumors) and optimal clearance from non-target tissues and blood, administration of 18 F labeled imaging probe 2. Construct 2 is intracellularly conjugated to entrapped 1 via a Staudinger ligation. After clearing of unbound 2, PET images can be recorded, delineating tumor location and activity.
Embodiment 2
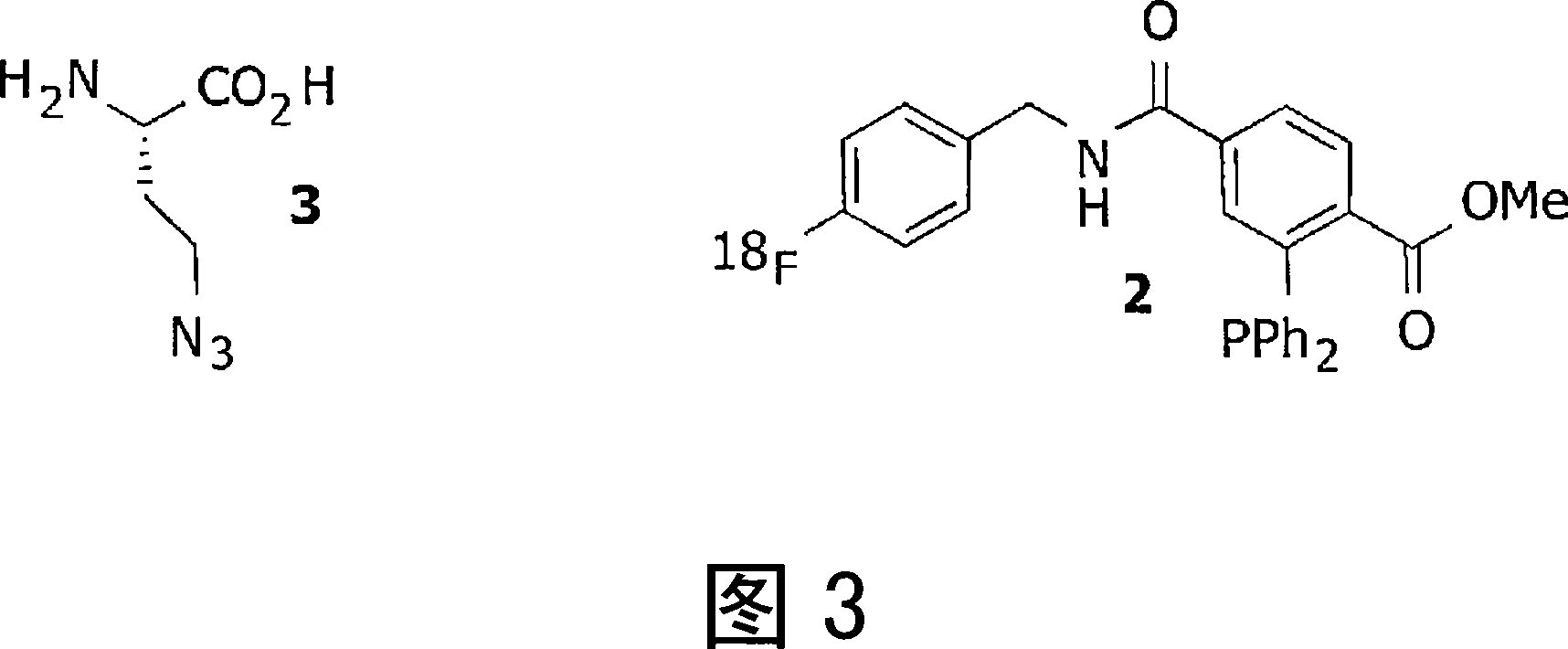
[0069] Example 2: Pretargeted Imaging of Tumors Using Azide-Amino Acids
[0070] Refer to Figure 3. The E. coli translation apparatus [bertozzi_PNAS2002] recognizes Azidohomoalanine (3) as a methionine substitute, which can serve as a metabolic / proliferation marker. Systemic administration of azidohomoalanine (3). Following optimal accumulation in tissues with high amino acid uptake (such as tumors) and optimal clearance from non-target tissues and blood, administration of 18 F labeled imaging probe 2. Construct 2 is intracellularly conjugated to entrapped 3 via a Staudinger ligation. After clearing of unbound 2, PET images can be recorded, delineating tumor location and activity.
Embodiment 3
[0071] Example 3: Pretargeted Imaging of Tumors Using Azide-Nucleosides
[0072] Refer to Figure 4.
[0073] Cell proliferation is increased in tumors, which leads to increased DNA replication and thus to an increased requirement for nucleosides. Given azide-modified thymidine 4, it is taken up by rapidly dividing cells. After optimal uptake in target cells, inject 18 F-labeled cyclooctyne compound 5, which binds to entrapped 4 via [3+2]azide-alkyne cycloaddition. After clearing of unbound 5, PET images can be recorded, delineating tumor location and activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com