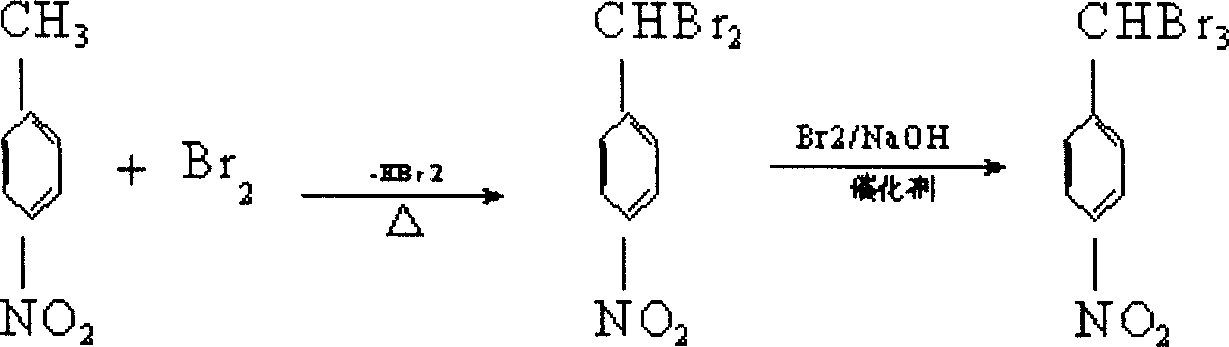Synthetic process of para-nitrotribromotoluene
A technology of nitrotribromotoluene and p-nitrotoluene, which is applied in the field of bromide synthesis technology, can solve the problems of harsh operating conditions, large bromine atom volume, and low product yield, and achieve low production cost and high product yield. High, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
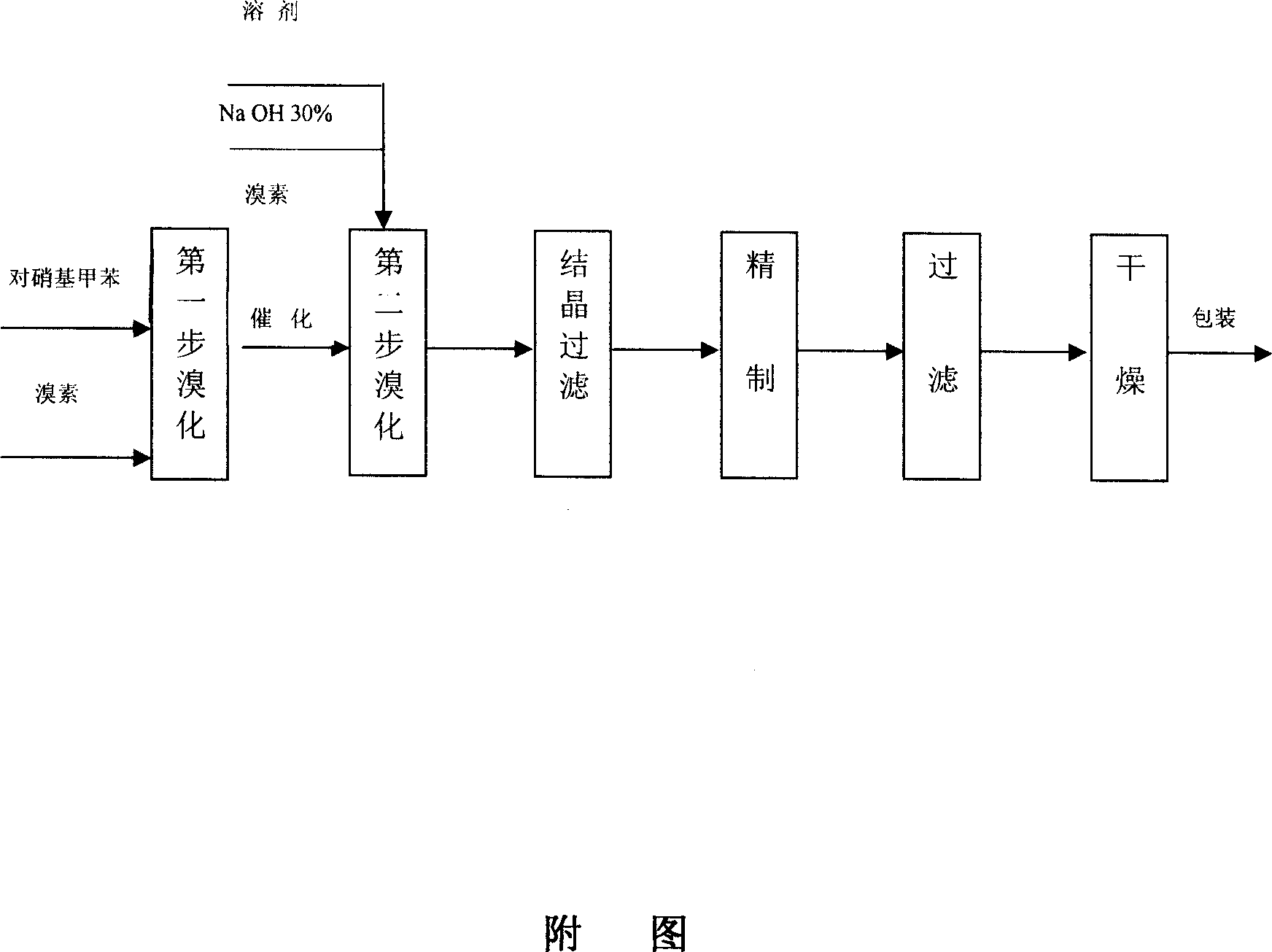
[0023] Example 1. A kind of synthesis technique of p-nitrobenzotribromotoluene, its step is as follows,
[0024] (1) The first step reaction: add p-nitrotoluene to the reaction kettle, open the tail gas absorption device, absorb the hydrogen bromide and excess bromine produced by the reaction with water; control the reaction temperature at 130°C, add bromine dropwise, and the reaction time Control within 3 hours; the reaction generates p-nitrodibromotoluene, and the temperature is lowered to 60°C;
[0025] (2) The second step reaction: put carbon tetrachloride, catalyst BTEAB, sodium hydroxide, and water into the above-mentioned reaction kettle in sequence, control the temperature at 40°C, then add bromine dropwise, and the reaction time is 2 hours, and the crude product is obtained by filtration ;
[0026] (3) Put the above crude product and ethanol into the reaction kettle, stir, heat up and reflux for 20 minutes, cool to room temperature, filter and dry to obtain the fini...
Embodiment 2
[0027] Example 2. A kind of synthesis technique of p-nitrobenzotribromotoluene, its step is as follows,
[0028] (1) The first step reaction: add p-nitrotoluene into the reaction kettle, open the tail gas absorption device, absorb the hydrogen bromide and excess bromine produced by the reaction with water; control the reaction temperature at 180°C, add bromine dropwise, and the reaction time Control within 8 hours; the reaction generates p-nitrodibromotoluene, and the temperature is lowered to 80°C; wherein, the molar ratio of p-nitrotoluene to bromine is: 1.0:2.0;
[0029] (2) The second step reaction: Put carbon tetrachloride, catalyst BTEAB, sodium hydroxide, and water into the above-mentioned reaction kettle in turn, control the temperature at 80°C, then add bromine dropwise, and the reaction time is 6 hours, and filter to obtain the crude product The molar proportioning ratio of the system is: p-nitrodibromotoluene: bromine: sodium hydroxide=1: 1.00: 1.10; the catalyst B...
Embodiment 3
[0031] Example 3. A kind of synthesis technique of p-nitrobenzotribromotoluene, its step is as follows,
[0032] (1) The first step reaction: add p-nitrotoluene to the reaction kettle, open the tail gas absorption device, absorb the hydrogen bromide and excess bromine produced by the reaction with water; control the reaction temperature at 150°C, add bromine dropwise, and the reaction time Control within 5 hours; react to generate p-nitrodibromotoluene, drop 70°C; wherein, the molar ratio of p-nitrotoluene to bromine is: 1.0:2.2;
[0033] (2) The second step reaction: Put carbon tetrachloride, catalyst BTEAB, sodium hydroxide, and water into the above-mentioned reaction kettle in sequence, control the temperature between 55°C, then add bromine dropwise, the reaction time is 4 hours, and filter Obtain crude product; The molar proportioning ratio of system is: p-nitrodibromotoluene: bromine: sodium hydroxide=1: 1.50: 1.60; Catalyst BTEAB consumption is 3% of p-nitrodibromotolue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com