Blood pressure depressed peptide from royal jelly
A technology of royal jelly and protein decomposition products, applied in the fields of food and oral preparations, to achieve the effect of effective prevention or treatment of hypertension, less side effects and strong ACE inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0316] Using 1 g of ethanol-denatured protein precipitated by adding ethanol to royal jelly, 250 mg of porcine trypsin was added and treated at pH 8.0 for 24 hours. After the reaction, heat at 100° C. for 10 minutes to inactivate the enzyme.
[0317] The reaction solution was adjusted to pH 4.5, applied to a synthetic adsorbent (Sepabis SP-70 (manufactured by Mitsubishi Chemical)), and washed with water to remove sodium chloride, acidic or alkaline free amino acids, sugars, etc. , and then eluted with ethanol to obtain the desalted protein hydrolyzate of the present invention.
[0318] The protein breakdown product after desalination does not substantially contain basic free amino acids, acidic free amino acids, alcoholic free amino acids, free Ala and free Gly, and the residual rate of Lys calculated according to Mathematical Formula 1 is about 45%, calculated according to Mathematical Formula 2 The content ratio (molar basis) of Leu was about 2.3 times. In addition, the su...
Embodiment 2
[0327] Embodiment 2: Structural analysis of the relevant components of royal jelly (RJ) protein hydrolyzate
[0328] 100 g of ethanol-denatured protein of royal jelly was taken, and enzymatically digested with porcine trypsin (pig-derived trypsin manufactured by Novozyme) at pH 8.0 and 37° C. for 24 hours. The reaction solution was heated in a boiling water bath for 30 minutes to inactivate the enzyme, then adjusted to pH 4.5 with hydrochloric acid, centrifuged (10000 rpm, 20 minutes), and the obtained supernatant was filtered with a membrane filter (0.8 μm), 1160 mL of RJ protein hydrolyzate was obtained.
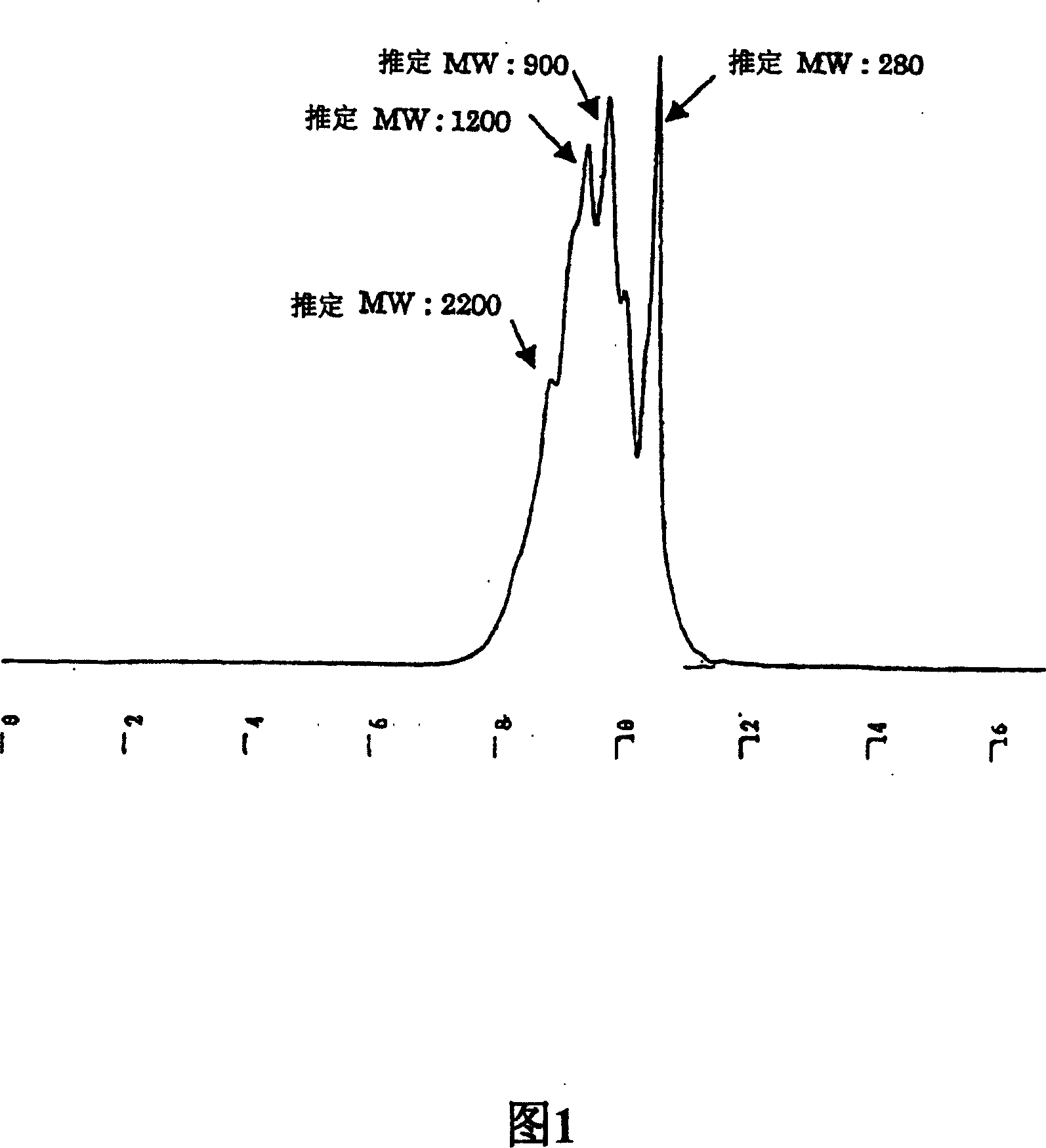
[0329] The obtained RJ protein hydrolyzate was eluted with water, 20%, 40%, 60%, 80% methanol, and methanol (100%) according to the above-mentioned flow chart 1 using Diayion HP20 to obtain Fraction 1 (Fr1) to Point 7 (Fr7).
[0330] Then, a detailed description of the isolation and identification of the 4 related components (peptides) identified from fractions 3-6 is as...
Embodiment 3
[0368] Example 3: Clinical trials of proteolytic products
[0369] In the same manner as in Example 1, a porcine trypsin hydrolyzate of RJ ethanol-denatured protein (containing 4 novel peptides of the present invention) was obtained, which was adsorbed on Diayion HP20 according to the above flow chart, and then washed to remove fraction 1 and 2, and then eluted with 80% ethanol to prepare the protein breakdown product of the present invention containing fractions 3-6, which were provided for the following experiments.
[0370] In order to study the antihypertensive effect and safety of long-term intake of tablets containing the obtained royal jelly protein hydrolyzate (RJPH), a group of people with high blood pressure (no treatment for normal high blood pressure patients and mild hypertensive patients) was carried out. A placebo-controlled double-blind trial. In order to make no difference in gender, age, systolic blood pressure, diastolic blood pressure, pulse rate, body wei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com