Fluoride modulated self-conversion method for preparing high active censpheres of titanium dichloride
A titanium dioxide, hollow microsphere technology, used in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
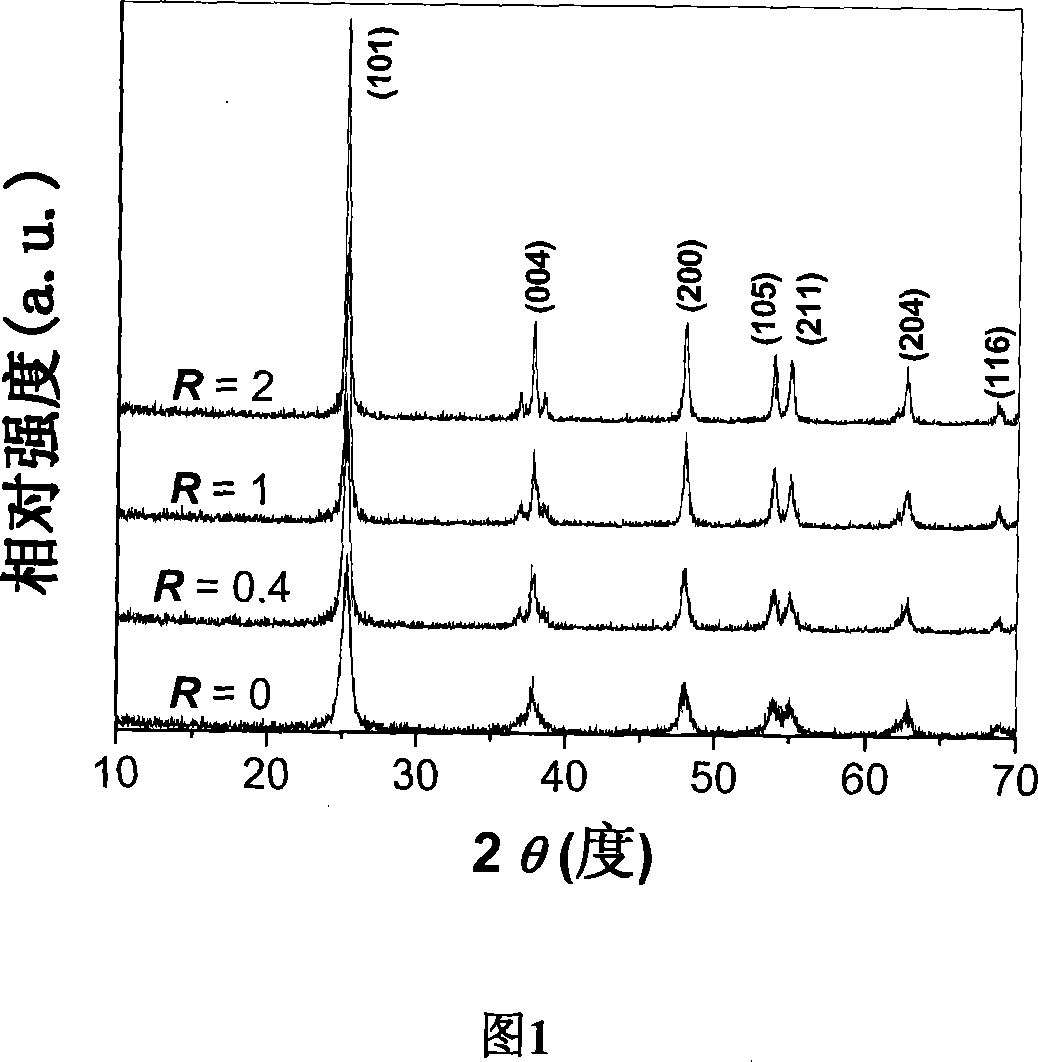
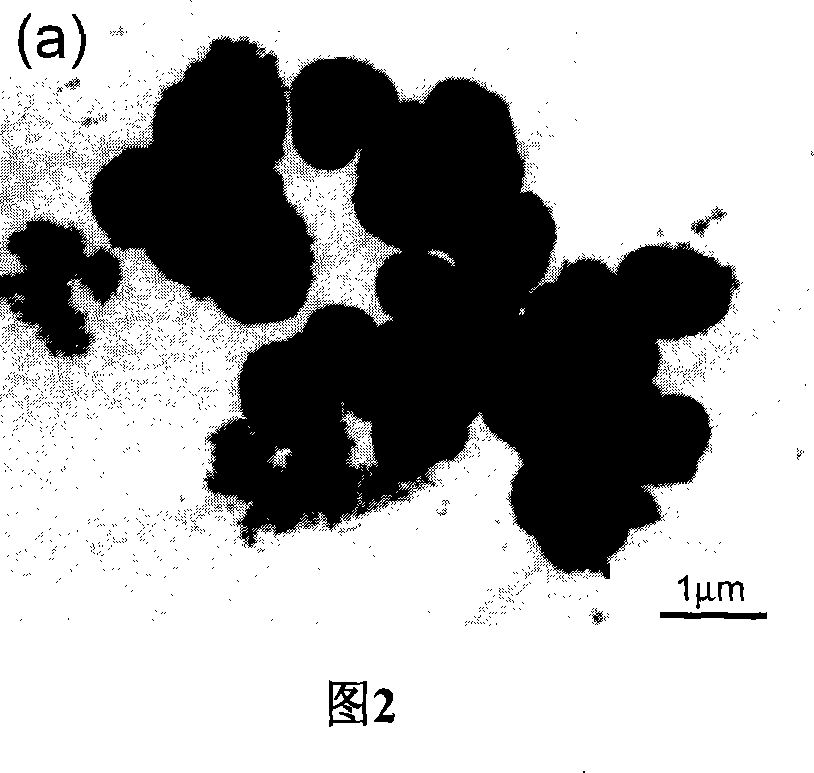
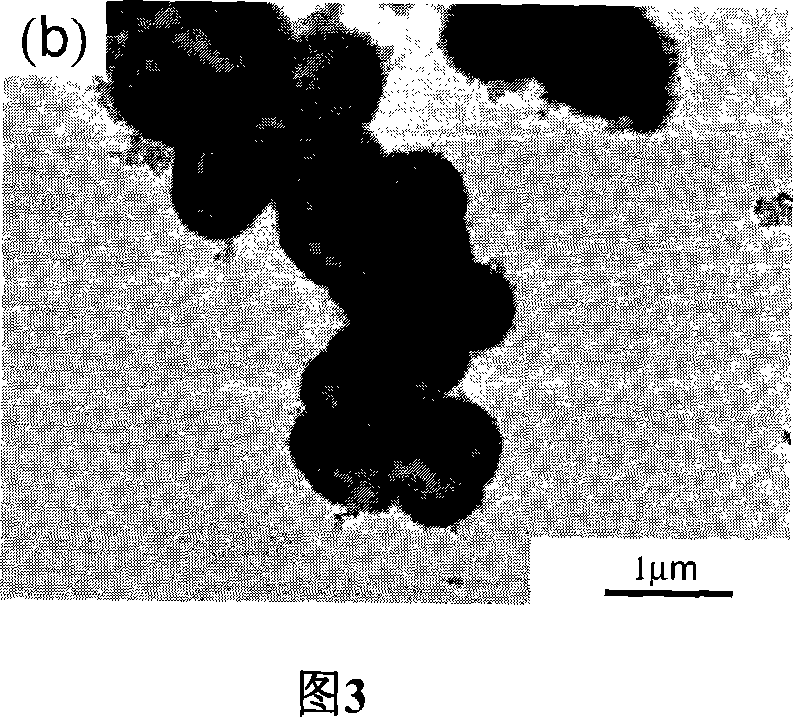
[0026] In order to prepare high-activity titanium dioxide hollow microspheres, titanium sulfate is used as titanium source. First, prepare a dilute sulfuric acid solution with a molar concentration of 1M, add titanium sulfate to the dilute sulfuric acid solution, and prepare a titanium sulfate sulfuric acid solution with a molar concentration of 0.5M. Then, a solution of ammonium fluoride with a molar concentration of 1M was prepared. The prepared acidic titanium sulfate solution and ammonium fluoride solution are uniformly mixed under magnetic stirring, and the ratio R of fluorine to titanium varies between 0 and 2 (R=0, 0.4, 1, 2). This mixed solution was transferred to a 100 ml hydrothermal kettle so that 80% of the volume of the hydrothermal kettle was filled. Cover the hydrothermal kettle tightly and keep it at 200°C for 9 hours to carry out the hydrothermal reaction. The white solid precipitate obtained from the reaction was collected and washed, then dried and kept at...
Embodiment 2
[0034] In order to test the effect of reaction time on the hollow structure of the sample, the reaction temperature and R were fixed at 200°C and 1, respectively. Except for the different reaction times, other reaction conditions such as the amount of titanium sulfate and ammonium fluoride were exactly the same as in Example 1. . The results showed that the samples prepared at 30 minutes were amorphous solid spheres. When the hydrothermal time was extended to 9 hours, as mentioned above, the product was hollow spheres of anatase phase. And the shell of the hollow ball becomes thinner and thinner with the prolongation of the water heating time. It is worth noting that the size of the particles increases slightly during the formation of the hollow spheres. With the prolongation of hydrothermal time, the average particle size gradually increased from 0.9 microns at 30 minutes to 1 micron at 9 hours and 1.2 microns at 36 hours. Based on the above results, the formation of hollo...
Embodiment 3
[0036] In order to test the influence of the reaction temperature on the hollow structure of the sample, the reaction time and R were fixed at 9 hours and 1 respectively, except that the reaction temperature was different, other reaction conditions such as: the amount of titanium sulfate and ammonium fluoride, etc. were exactly the same as in Example 1 . The results show that the samples prepared at the reaction temperature lower than 100℃ are solid spheres. When the reaction temperature is higher than 100°C, the product is an anatase phase hollow sphere. When the reaction temperature is higher than 200°C, the prepared product is an anatase phase hollow sphere, but it is easy to break.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com