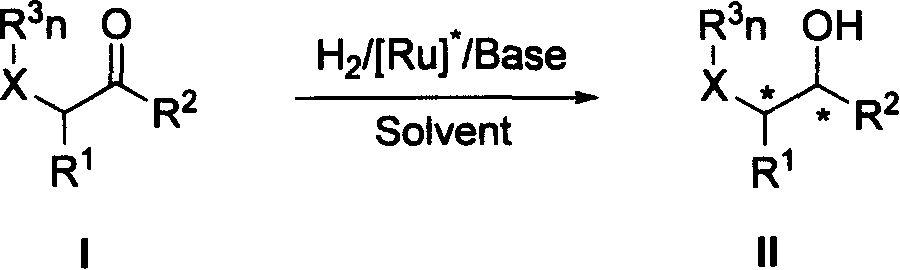Method of preparing chiral primary alcohol and secondary alcohol with chirality center at ortho position of hydroxyl group
A chiral, ortho-position technology, applied in the preparation of hydroxyl compounds, organic compounds, chemical instruments and methods, etc., to achieve the effects of high reactivity, high conversion number, and high optical yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
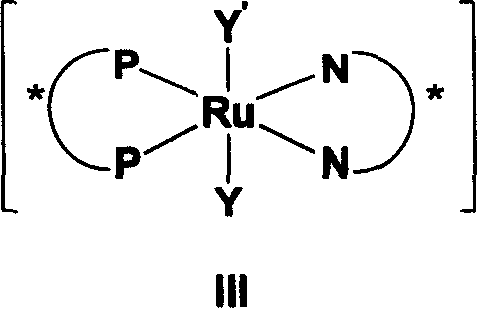
[0020]The method for preparing chiral primary alcohols and secondary alcohols having a chiral center at the ortho position of the hydroxyl group described in the present invention is realized by a chiral bisphosphine-ruthenium-bisamine catalyst having the general formula III under the condition of the presence of a base. The catalyst includes a chiral bisphosphine ligand with a general formula IV with a spiro ring skeleton and a well-known bisphosphine ligand with a general formula V with a biaryl ring skeleton. The chiral diamine used in the catalyst is a chiral diamine compound with general formula VI.
[0021]
[0022] In formula III,
[0023] Y and Y' are respectively Cl, Br, I or H, and Y and Y' may be the same or different.
[0024] Chiral bisphosphine ligands shown in general formula III include chiral bisphosphine compounds having general formulas IV and V.
[0025]
[0026] In formulas IV and V,
[0027] n=0~4; R 1 , R 2 for H, C 1 ~C 10 Alkyl, C 1 ~C 1...
Embodiment 1
[0042] Preparation of chiral bisphosphine-ruthenium-bisamine catalyst ((S)-7,7'-bis-(bis-(4-methoxy-3,5-dimethylphenyl)-phosphino)-1 , 1′-spirodihydroindane (DMM-SDP) and (R, R)-1,2-cyclohexanediamine ((R, R)-DACH) and [RuCl 2 (C 6 h 6 )] 2 Complexation preparation (RuCl 2 -(S)-Xyl-SDP / (R,R)-DACH) as an example)
[0043] In a nitrogen atmosphere, (S)-7,7'-bis-(bis-(4-methoxy-3,5-dimethylphenyl)-phosphino)-1,1'-spirodihydroindane (DMM-SDP) (141.9mg, 0.165mmol) and [RuCl 2 (C 6 h 6 )] 2 (40mg, 0.08mmol) was dissolved in 3mL DMF, heated to 100°C, stirred for 3.0 hours, after a little cooling, added (R,R)-1,2-cyclohexanediamine ((R,R)-DACH) (18.8 mg, 0.165 mmol). After stirring at room temperature for 18 hours, the solution was removed in vacuo to obtain a reddish-brown solid. The solid was used directly in the hydrogenation reaction.
[0044] The other chiral bisphosphine-ruthenium-bisamine catalysts were prepared in the same way.
Embodiment 2
[0046] Asymmetric Catalytic Hydrogenation of α-Aryl Aldehydes to Synthesis of Chiral β-Aryl Primary Alcohols
[0047] Add 0.002mmol of catalyst, 2mmol of α-arylaldehyde, 0.04mmol of potassium tert-butoxide and 8mL of isopropanol into a hydrogenation reactor, and stir the hydrogenation reaction at room temperature for 8-24 hours under a hydrogen pressure of 50 atm. The solvent was removed under reduced pressure, and the target product was obtained through silica gel column chromatography. The conversion rate and optical purity of the product were analyzed by GC or HPLC, and the results are shown in Table 1.
[0048] Table 1.
[0049]
[0050] α-Arylaldehyde
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com