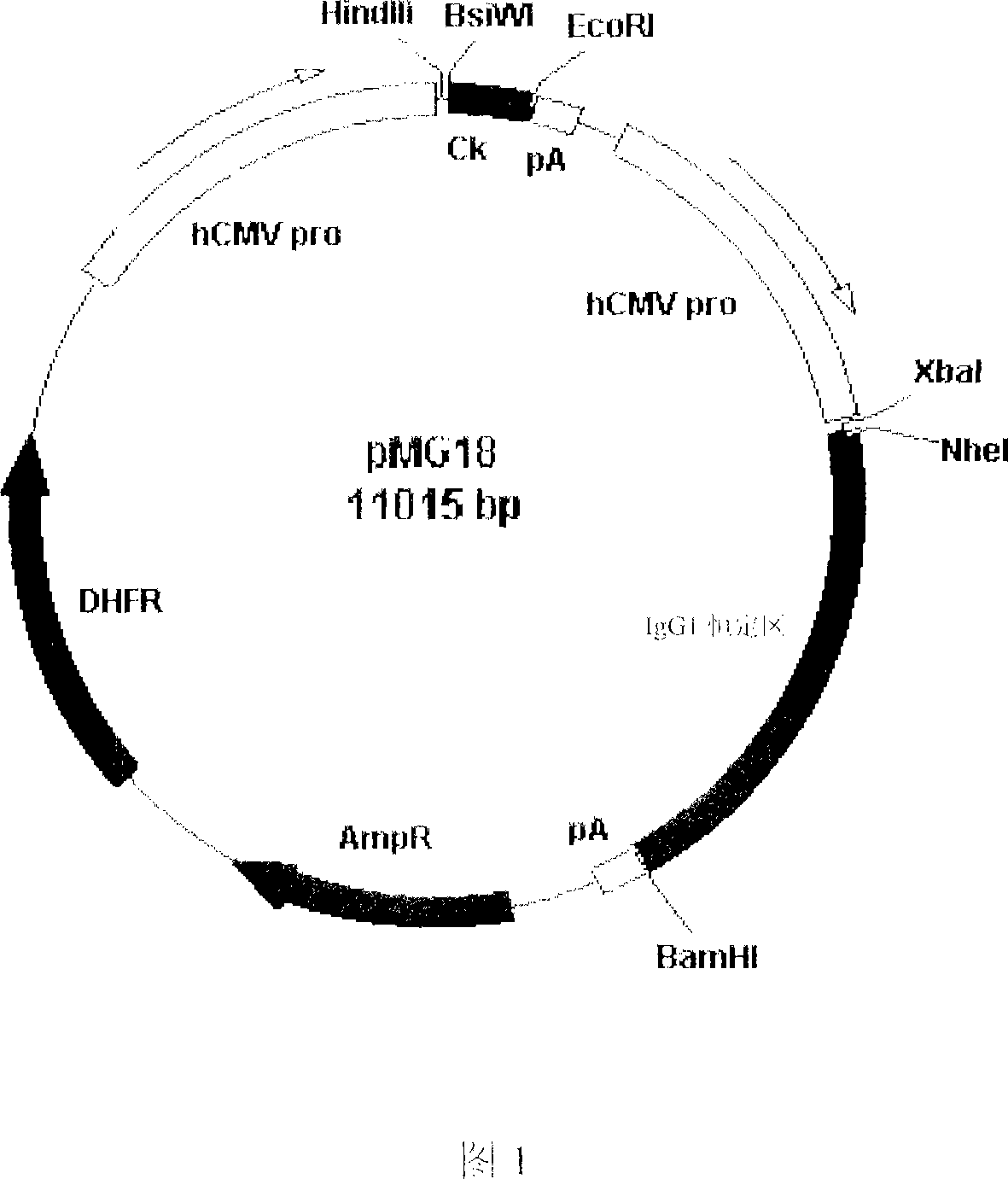
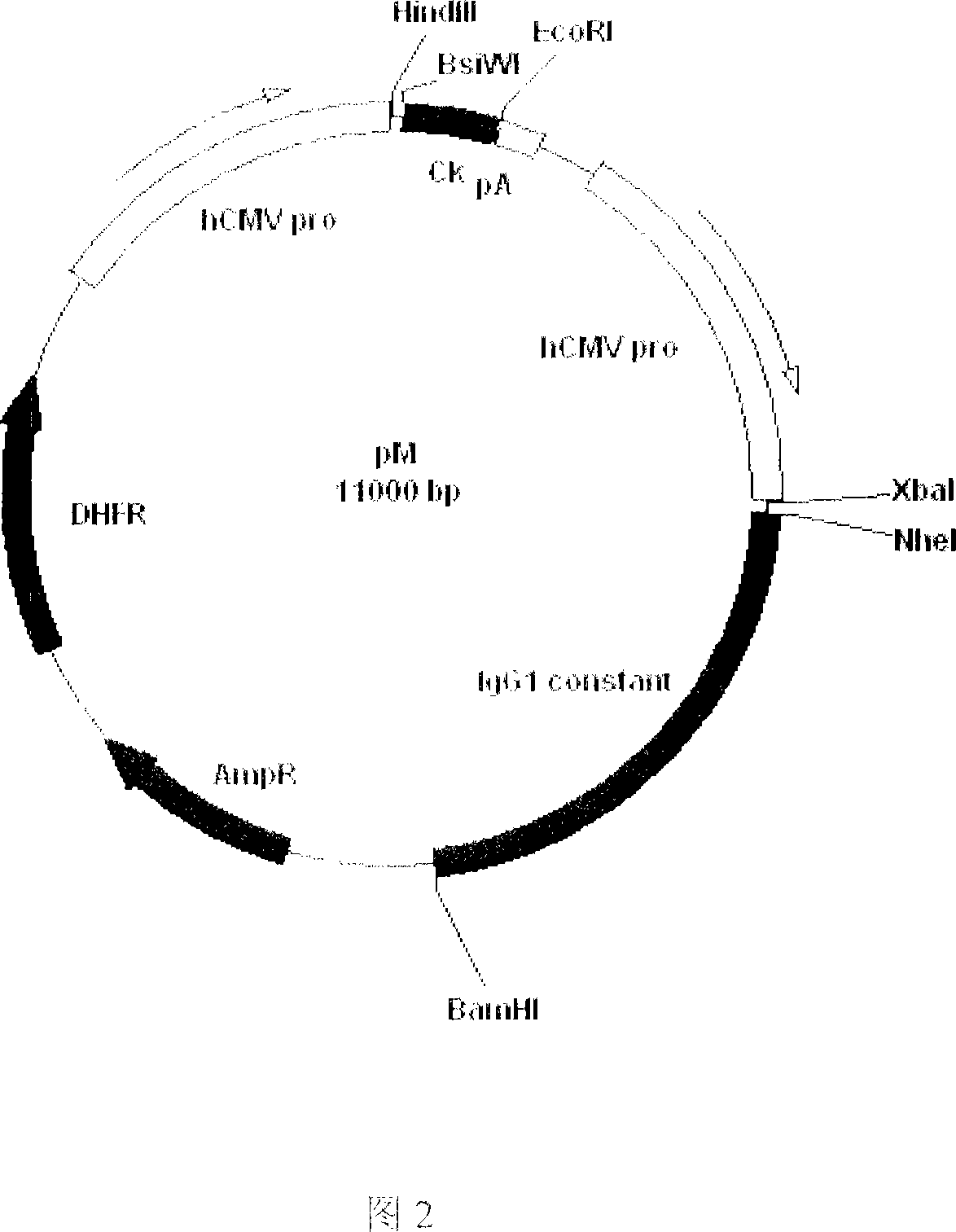
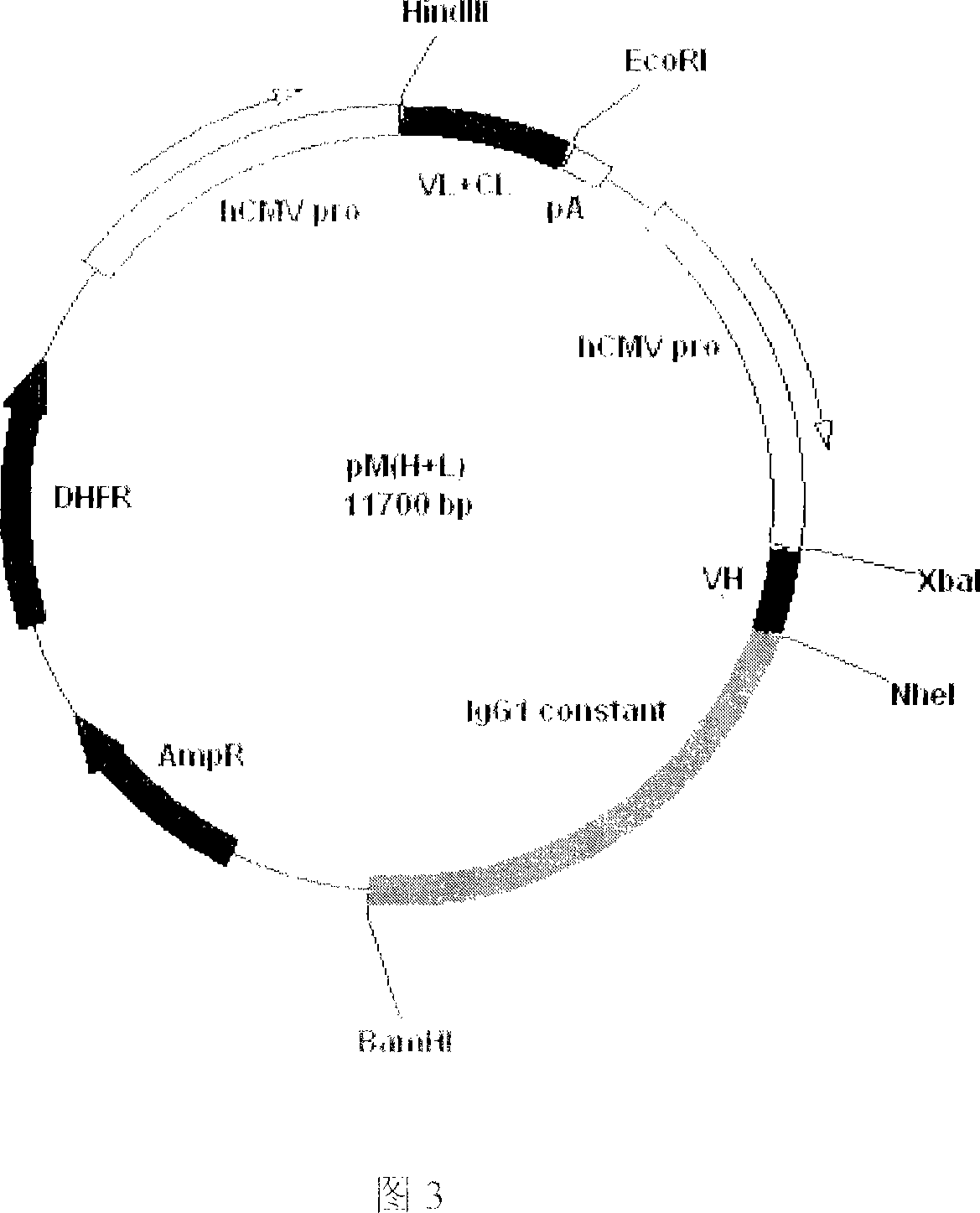
Recombinant anti-CTLA4 monoclonal antibody, its production and use
A monoclonal antibody, heavy chain technology, applied in recombinant DNA technology, botanical equipment and methods, biochemical equipment and methods, etc., can solve problems such as systemic reactions, toxic and side effects, and achieve high sensitivity and specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Screening the variable region of cytolytic T cell-associated antigen 4 antibody gene from the antibody library
[0042] 1) Construction of mouse antibody library
[0043] CTLA4-Fc fusion protein (R&D, 325-CT / CF) and Freund's adjuvant were used to immunize Balb / C mice. After four times of immunization, the mouse serum was diluted 1:500 and showed a strong positive reaction with CTLA4. Mouse spleen according to Marks et al. J. Mol. Biol., 222, 581-597. Hoogenboom and Winter, J. Mol. Biol., 227, 381-388. Haidaris CG et al. J Immunol Methods. 2001 Nov 1; 257( 1-2): 185-202, Griffiths, A.D. et al. EMBO J., 13, 3245-3260 (1994). The method described in Nissim, A. et al. EMBO J., 13, 692-698 (1994), Construction of murine antibody library.
[0044] 2) screening
[0045] Add 1 ml of the revived antibody library strain to 14 ml of fresh LB medium, and culture in a 50 ml Erlenmeyer flask at 37°C for 16 hours.
[0046] Centrifuge at a high speed of 12000rpm for 10 mi...
Embodiment 2
[0065] Example 2 Expression Intensity of Antibody Genes in CHO Cells
[0066]The 12 high-expression candidate clones obtained from the above screening were cultured in a 10 cm tissue culture dish, and the expression level of the antibody was determined by ELISA as described below. Coat goat anti-mouse IgG (Fc) on an ELISA plate, overnight at 4°C, block with 2% BSA at 37°C for 2 hours, add the culture supernatant to be tested and a standard (mouse IgG1), incubate at 37°C for 2 hours, add HRP-goat anti-mouse IgG (κ)) for binding reaction, incubated at 37°C for 1 hour, added TMB at 37°C for 10 minutes, and finally washed with H 2 SO 4 Termination reaction, test A 450 value. Table 1 below shows the expression levels of the 12 candidate clones obtained from the above screening.
[0067] Cell line number
[0068] As can be seen from Table 1 above, 3C4, 1A2 and 8H6 have very high expression levels.
Embodiment 3
[0069] Example 3 DNA sequencing of anti-CTLA4 monoclonal antibody gene
[0070] According to the pedigree, DNA sequencing was performed on the anti-CTLA4 antibody gene of the 8H6 cell line obtained above. The results are as follows: SEQ ID NO: 3 shows the 8H6 light chain variable region gene sequence (5' to 3') 336bp, and its deduced amino acid sequence is shown in SEQ ID NO: 1; SEQ ID NO: 4 shows the 8H6 heavy chain Variable region gene sequence ((5' to 3') 363 bp), its deduced amino acid sequence is shown in SEQ ID NO:2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com