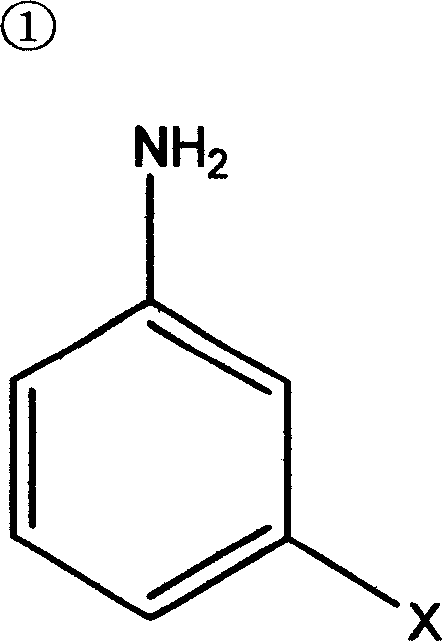
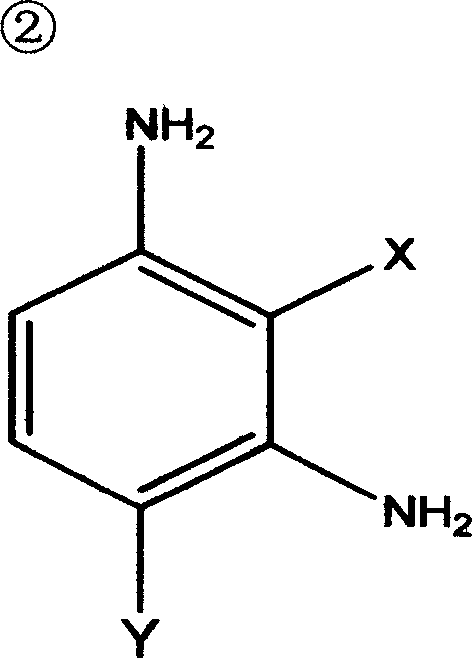
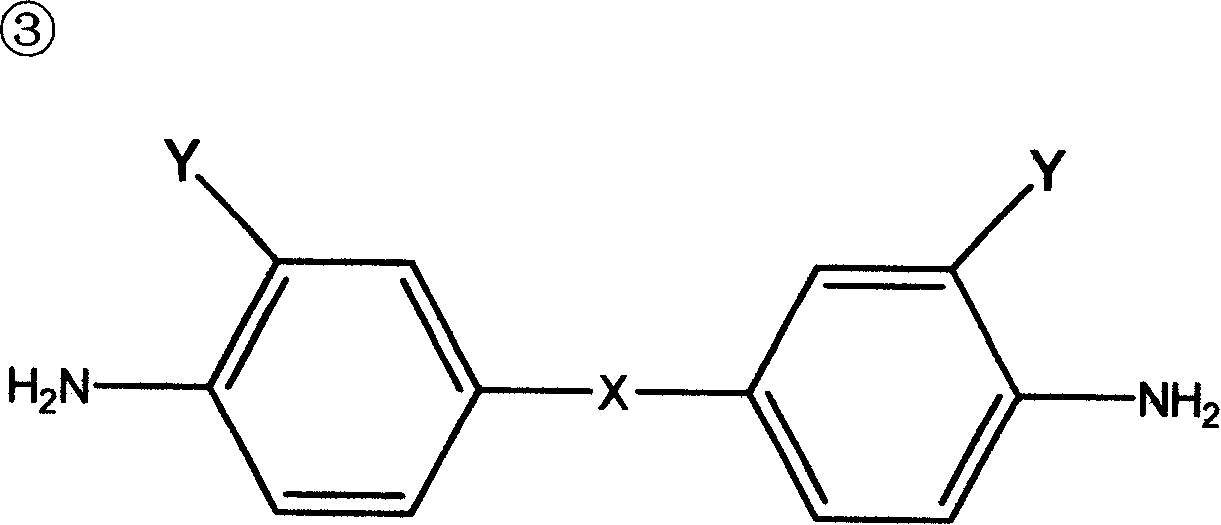
Synthesis of polyaspartate aminoester polyether-amine containing secondary-amine group
A technology of polyaspartic acid ester and synthesis method, which is applied in the field of synthesis of polyaspartic acid ester and polyetheramine, can solve the problem of not mentioning time and the like, and achieve the effects of shortening the reaction time and prolonging the gel time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The four-neck flask was equipped with a stirrer, heater, addition funnel and nitrogen inlet. With 400g (0.400 equivalent) of Jeffamine D-2000 and 19.8g (0.200 equivalent) of 4,4'-diaminodiphenylmethane (MDA), the flask was heated up to 120 ° C, and was added to the flask through the addition funnel within 4 hours. 136.8 g (0.600 equivalents) of dibutyl maleate were added. After the dropwise addition, the reaction was continued at this temperature for 30 hours, and the reaction was left at room temperature for 3 months to reach 100% completion. The gel time of its reaction with N3390 curing agent reaches 4 hours.
Embodiment 2
[0061] The four-neck flask was equipped with a stirrer, heater, addition funnel and nitrogen inlet. With 400g (0.400 equivalent) of Jeffamine D-2000 and 26.4g (0.267 equivalent) of 4,4'-diaminodiphenylmethane (MDA), the flask was heated to 120 ° C, and was added to the flask through the addition funnel within 4 hours. 152.1 g (0.667 equivalents) of dibutyl maleate were added. After the dropwise addition, the reaction was continued at this temperature for 30 hours, and the reaction was left at room temperature for 4 months to reach 100% completion. The gel time of its reaction with N3390 curing agent reaches 3.5 hours.
Embodiment 3
[0063] The four-neck flask was equipped with a stirrer, heater, addition funnel and nitrogen inlet. Put 400g (0.400 equivalent) of Jeffamine D-2000 and 17.8g (0.200 equivalent) of diethyltoluenediamine (DETDA) into the flask, raise the temperature of the flask to 125°C, and add 136.8 g (0.600 equivalents) of dibutyl maleate. After the dropwise addition, the reaction was continued at this temperature for 30 hours, and the reaction was left at room temperature for 3 months to reach 100% completion. The gel time of its reaction with N3390 curing agent reaches 3.5 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com