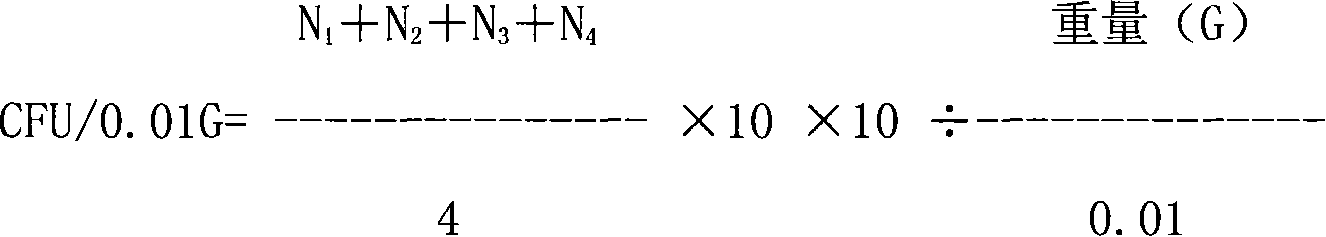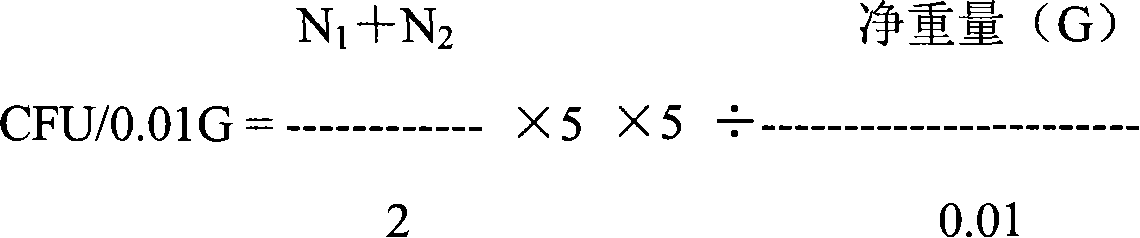Quality control standard substance for microbe detection and its preparation method
A technology for microbial detection and control standards, applied in the direction of microbial-based methods, biochemical equipment and methods, microbial measurement/inspection, etc., which can solve problems such as uncertainty in test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The microorganisms are adapted for 2 to 4 hours on the bacterial spore formation medium or fungal spore formation medium (the culture temperature of Bacillus subtilis var. The culture temperature of spores is 54°C to 56°C, and the culture temperature of Candida albicans is 25°C), the microorganisms enter the logarithmic growth phase, the number of microorganisms increases greatly, the microorganisms grow vigorously, the nutrients in the medium are exhausted, and the medium can be used The ingredients are greatly reduced, and the harmful substances produced are greatly increased. Microorganisms are cultured for more than 10 days in such a harsh environment where nutrients are insufficient and harmful substances exist, and more than 90% of microorganisms form spores and spores and enter a dormant state.
[0028] Wash the bacterial lawn on the culture medium with sterilized distilled water, mix thoroughly to form a 2.0×10 9 cfu / ml bacterial suspension, placed in a sterili...
Embodiment 2
[0095] Example 2 Used for synchronous positive control in the detection process of colony counting items
[0096] a. Take a "quality control standard substance for microbiological testing".
[0097] b, in the microbiological testing laboratory (biosafety protection laboratory). Open the plastic tube.
[0098] c. Take a pipette, draw 1.0ml of sterilized distilled water, add it into a plastic tube, and mix well.
[0099] d. Take another pipette, suck out the liquid in the plastic tube, and add it to the sterilized medium test tube (tube No. 1).
[0100] e. Continue to draw 1.0ml of sterilized distilled water, add it to the plastic tube, rinse the plastic tube, then suck out the sample liquid, and place it in the above-mentioned pilot test tube. Repeat this method 2 more times, fully wash the sample in the plastic tube, and place it in the pilot test tube.
[0101] f, continue to add sterilized distilled water into the test tube, the total amount is 5.0ml.
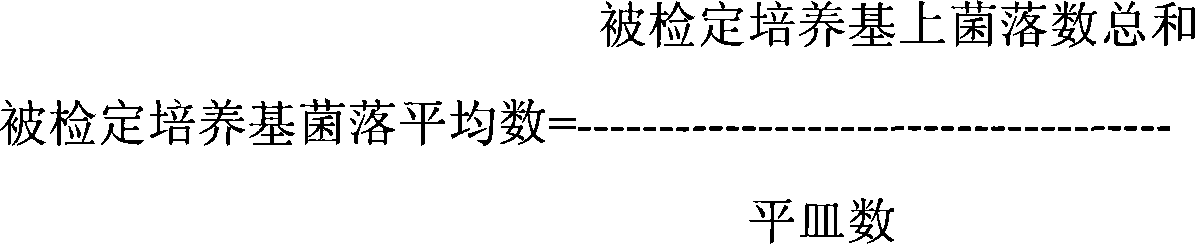
[0102] g, take a...
Embodiment 3
[0109] Example 3 Medium (nutrient agar) quality test
[0110] a. Take a "quality control standard substance for microbiological testing".
[0111] b. In the microbiological testing laboratory (biosafety protection laboratory). Open the plastic tube.
[0112] c. Take a pipette, draw 1.0ml of sterilized distilled water, add it into the plastic tube, and mix well.
[0113] d. Take another pipette, suck out the liquid in the plastic tube, and add it to the sterilized test tube.
[0114] e. Continue to draw 1.0ml of sterilized distilled water, add it to the plastic tube, rinse the plastic tube, then suck out the sample liquid, and place it in the above-mentioned pilot test tube. Repeat this method 2 more times, fully wash the sample in the plastic tube, and place it in the pilot test tube.
[0115] f, continue to add sterilized distilled water in the test tube, the total amount is 5.0ml, fully mix with a 1ml pipette (or with a mixer).
[0116] g. Use a 100ul pipette to absorb ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com