Crosslinking wrapped core slice in vivo for
A colon localization and drug delivery technology, applied in the directions of drug delivery, antitumor drugs, drug combination, etc., can solve the problems of unstable nature, difficult to popularize and use, difficult to control the degree of cross-linking, etc. stagnant release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
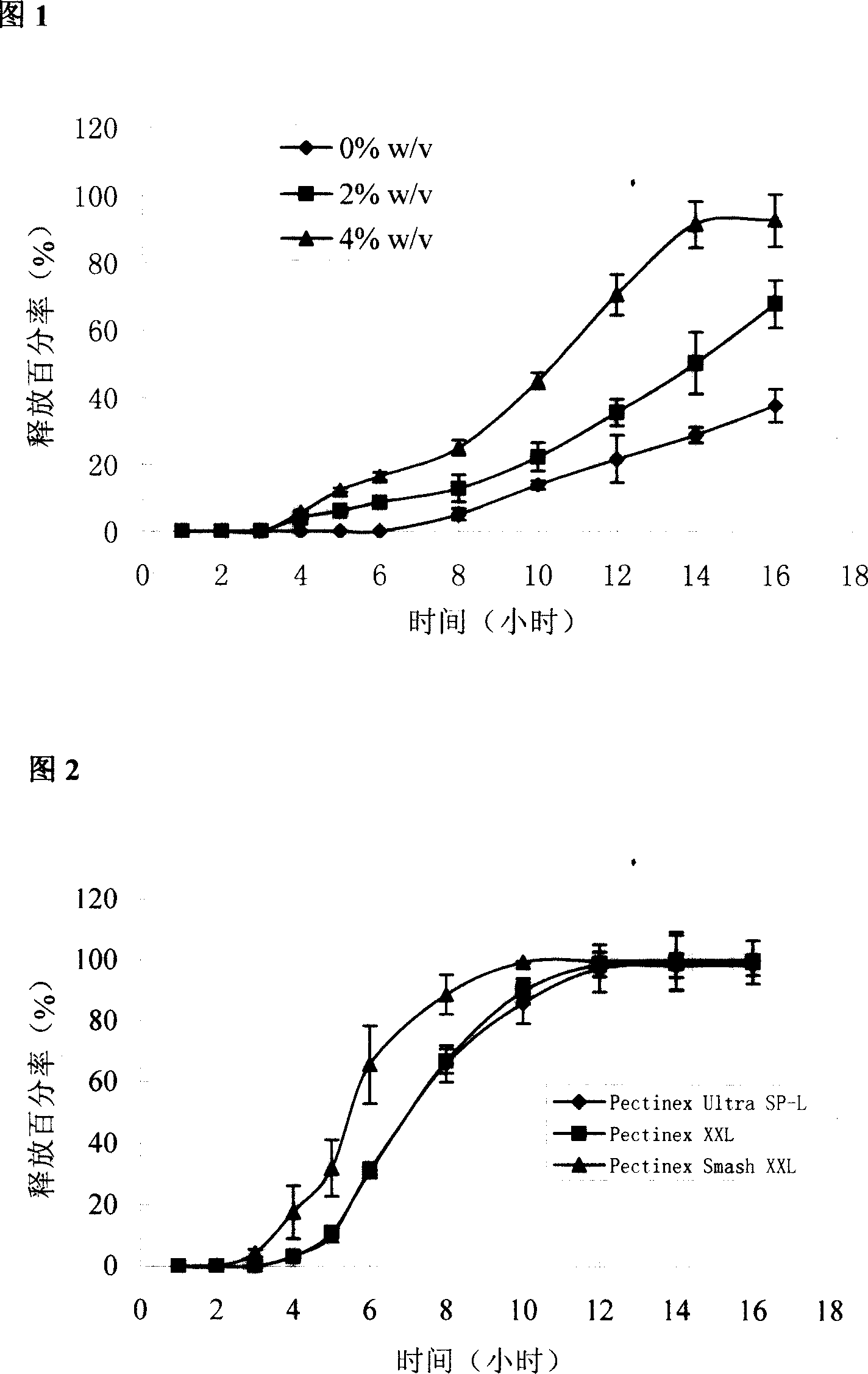
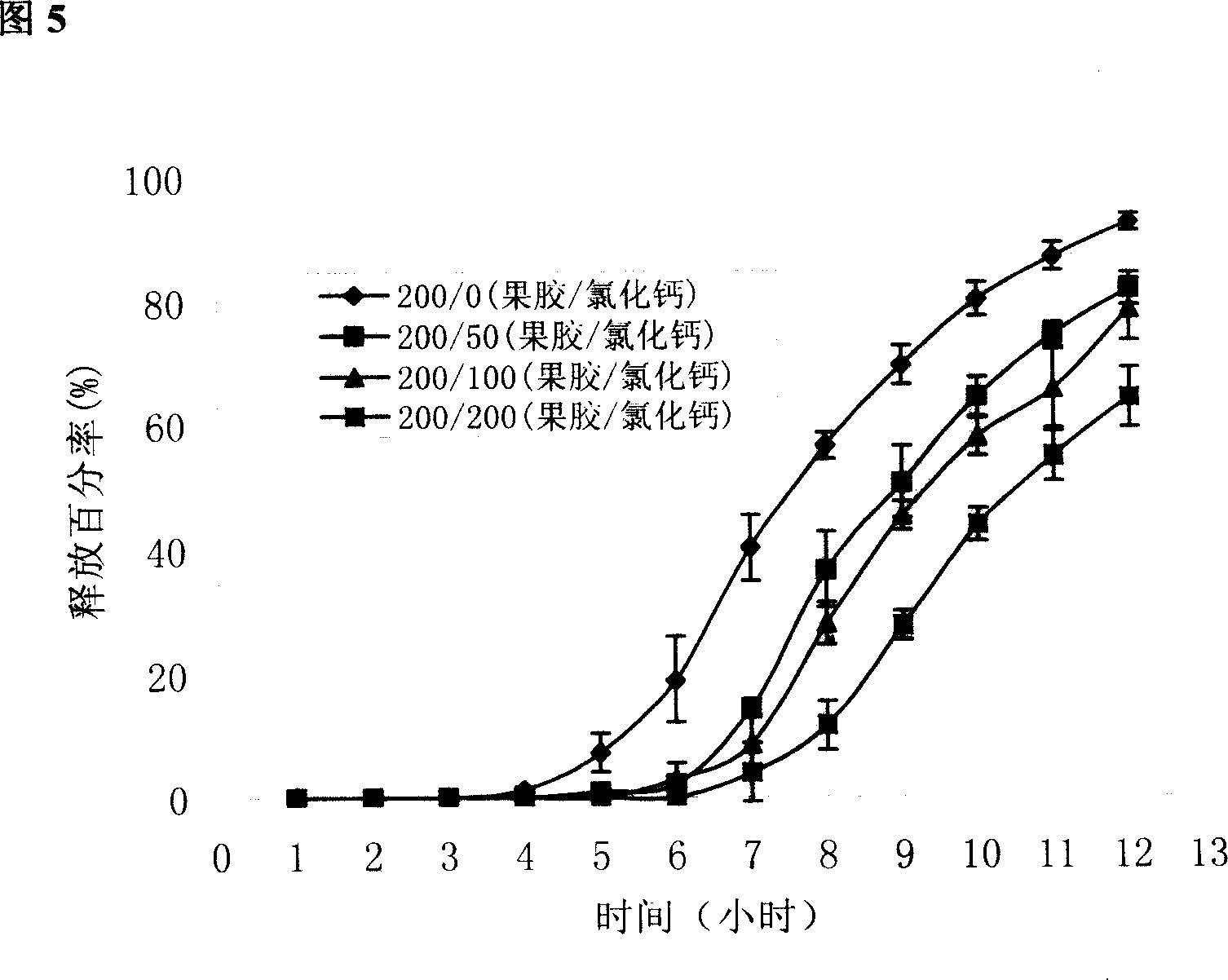
[0021] Example 1: Indomethacin pectin / calcium chloride in vivo cross-linked pressed package chip
[0022] Tablet prescription:
[0023] Indomethacin 25mg
[0024] Microcrystalline Cellulose 20mg
[0025] Lactose 20mg
[0026] Crospovidone 5mg
[0028] Scale up by 1000 pieces.
[0029] Preparation process: Indomethacin is in micronized form, with an average particle size of less than 5 microns, magnesium stearate is used directly, and other auxiliary materials are passed through an 80-mesh sieve. Precisely weigh the prescribed amount of indomethacin and various auxiliary materials, mix them evenly in a small mixer, and directly compress them into tablet cores with a diameter of about 6 mm on a ZDY-1 single-punch tablet press. Check by the relevant regulations in the two appendices of the 2005 edition of the Pharmacopoeia of the People's Republic of China, the tablet weighs 71 mg, the disintegration time is less than 30 seconds, the friabili...
Embodiment 2
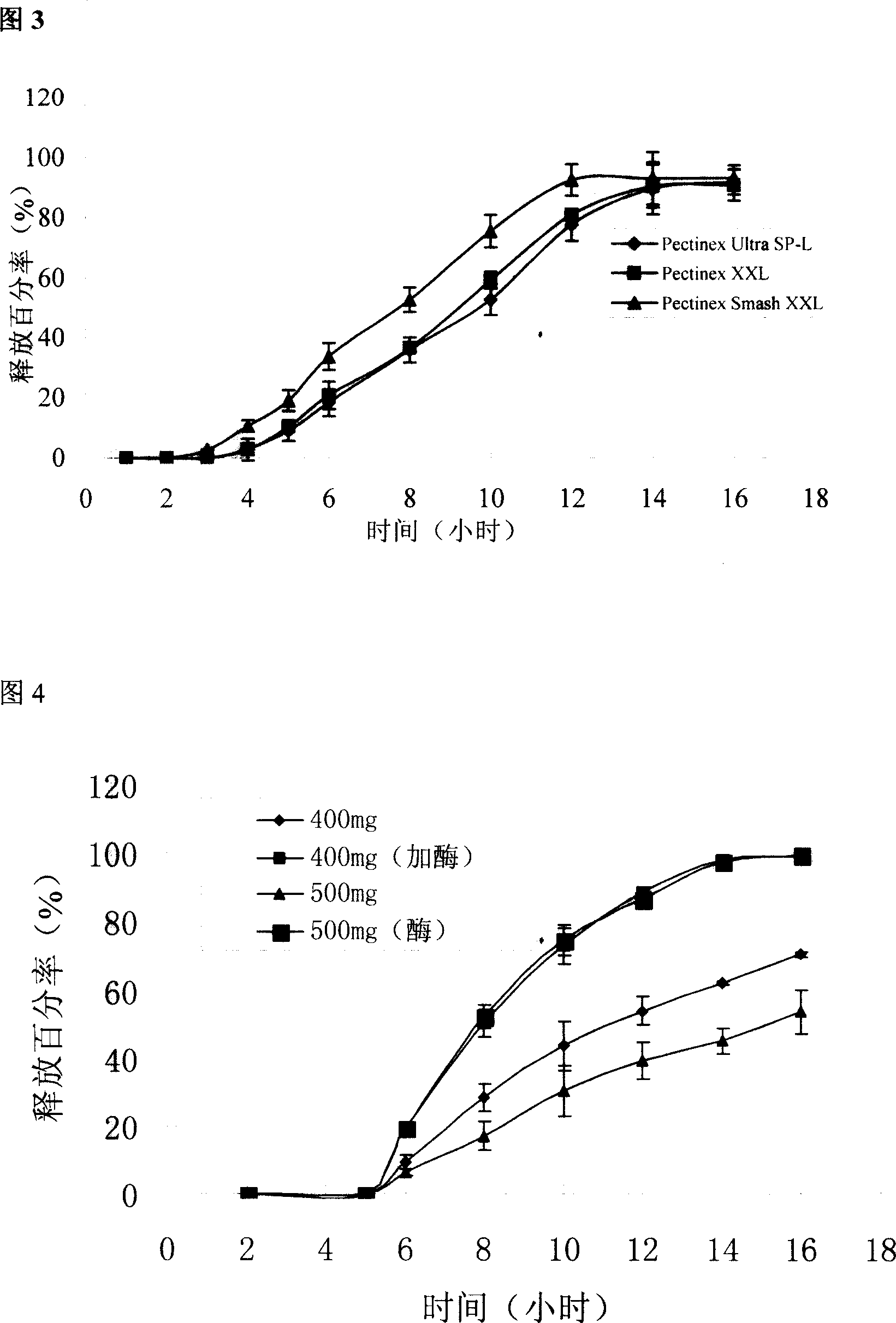
[0048] Example 2 Release of different pectin / calcium chloride ratio-coated chips
[0049] In order to further elucidate the in vivo cross-linking effect of calcium chloride on pectin, the release of coated chips was compared when the coating layer contained different ratios of pectin / calcium chloride.
[0050] Tablet core prescription is with embodiment 1.
[0051] The coating material prescription is shown in Table 2. Prepare according to the method for preparing the chip in Example 1. Prepared according to the prescription quantity of 1000 tablets. Check according to the relevant methods in the two appendices of the 2005 edition of the Pharmacopoeia of the People's Republic of China.
[0052] Formulation and basic properties of cake-packed tablets when the coating material contains different amounts of calcium chloride in table 2
[0053]
prescription number
Coating composition (mg)
Sheet weight
(mg)
tensile strength
(kg)
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com