Codon-optimized HPV16LI for salmonella vaccine strains against human papillomavirus type 16
An HPV16L1 encoding technology, applied in the field of HPV16L1 with optimized codons for SALMONELLA vaccine strains against human papillomavirus type 16, can solve the problem of difficulty in obtaining vaccines and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Plasmid construction and bacterial strains used
[0049] The L1S gene was synthesized by Microsynth, Buchs, Switzerland. The open reading frame is flanked at the 5' by Nco I restriction sites and at the 3' by Hind III restriction sites. This L1S Nco I-Hind III fragment was inserted to replace the original L1 Nco I-Hind III fragment in plasmid pFS14nsd HPV16-L1 (31). The resulting plasmid, pFS14nsd HPV16-L1S, was introduced into attenuated Salmonella enterica serovar Typhimurium strain PhoP by electroporation (37) c , (CS022(27)) and PhoP″(CS015(26)), both gifts from John Mekalanos, Boston, USA, x 4989 {Acya Acrp, (4)), x 4990 (Acya Acrp-cdd, (4)) and AaroA (SL7207(16)), a kind gift from Irene Corthesy-Theulaz, Lausanne, CH.
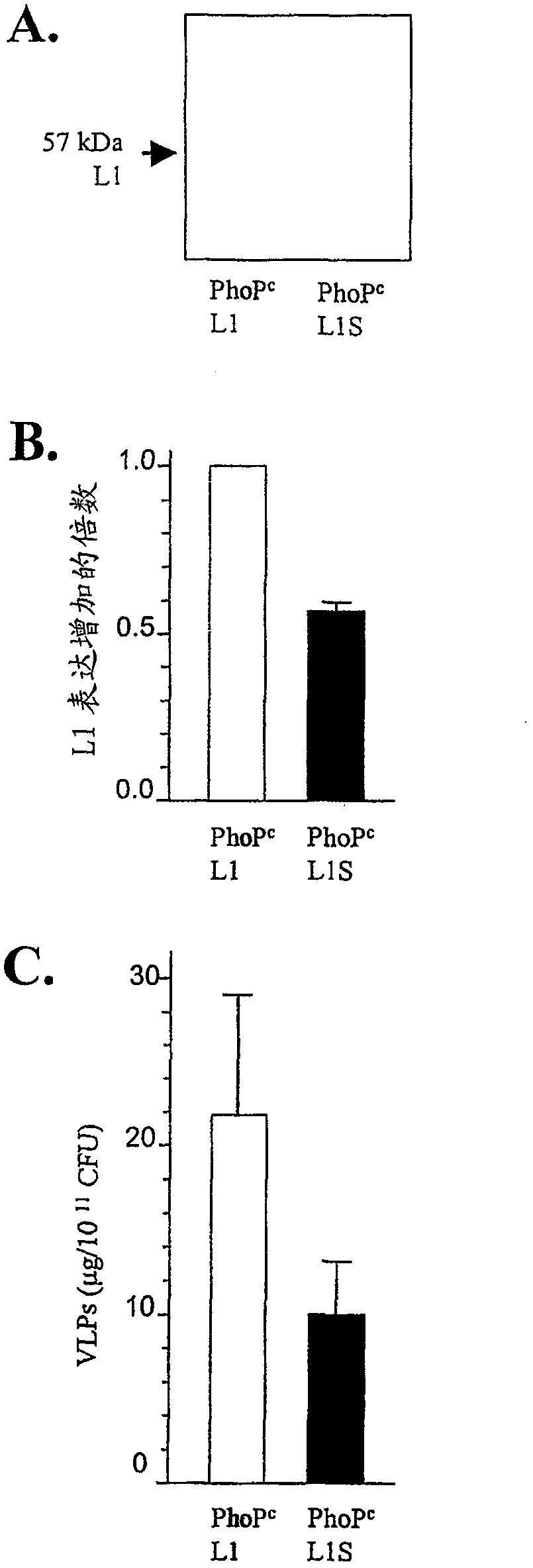
[0050] HPV16L1 and VLP analysis
[0051] Expression of L1 in Salmonella lysates was analyzed by Western blot using the anti-HPV16 L1 mAb, CAMVIR-1 (Anawa), as previously described (15, 31 ). Data were normalized to the level in bacteria as me...
Embodiment 2
[0063] Plasmid construction and bacterial strains used
[0064] In plasmid pFS14nsd HPV16-L1S, the ampicillin resistance coding sequence was replaced with the kanamycin resistance coding sequence as follows. The SacII-XbaI fragment containing the kanamycin coding sequence and promoter was generated by PCR using pET-9a (Novagen) plasmid DNA as a template. The primer used was a 25-mer primer located 54 nucleotides upstream from the first ATG of kanamycin and containing a SacII restriction site (underlined): 5'-GGG CCGCGG TGGTCATGAACAATAA-3', and a 28-mer primer containing an XbaI restriction site (underlined): 5'-GGG TCTAGA AGCTGTCAAACATGAGAAT-3'. Another large SacII-XbaI fragment containing the entire pFS14nsd HPV16-L1S plasmid sequence but without the ampicillin resistance gene was generated by inverse PCR using Expand High Fidelity PCR (Roche Molecular Biochemicals), using the following primers: 28-mer primer 5'-GGG located 92 nucleotides upstream from the ATG of ampicil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com