Method for preparing optical activity 2,3-dihydroxy teinai hemiacetal derivant
An internal hemiacetal, optically active technology, used in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of harsh reaction conditions, expensive reagents, and decreased yield, and achieve mild process conditions and optical purity. High, easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
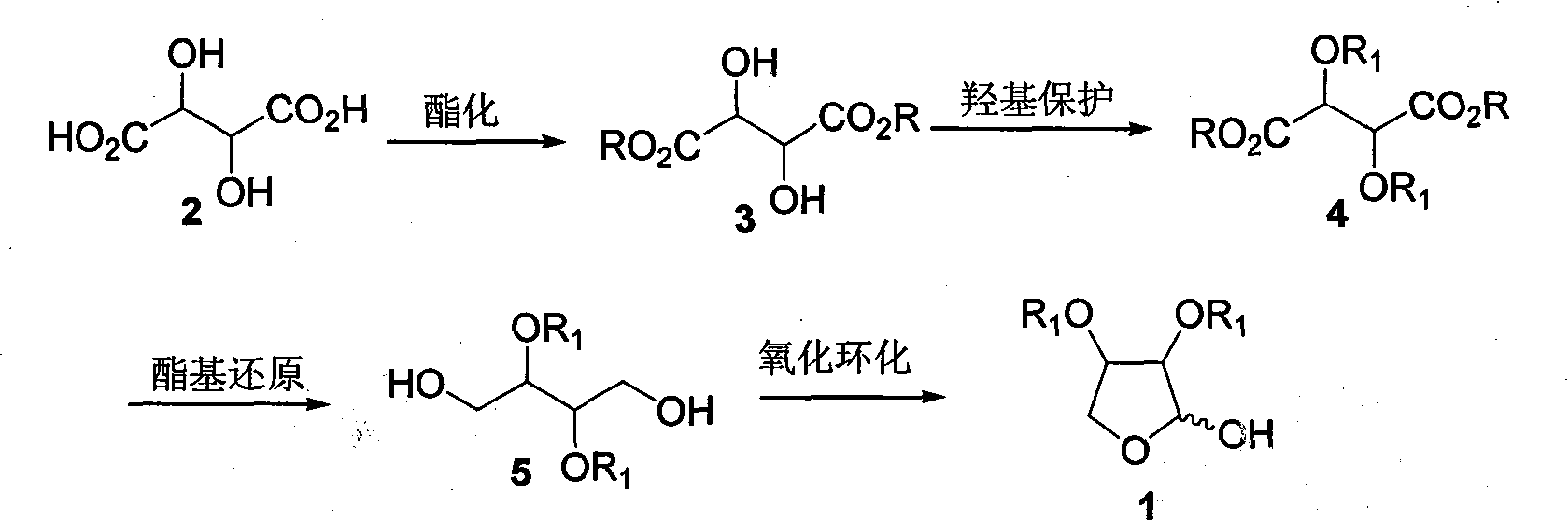
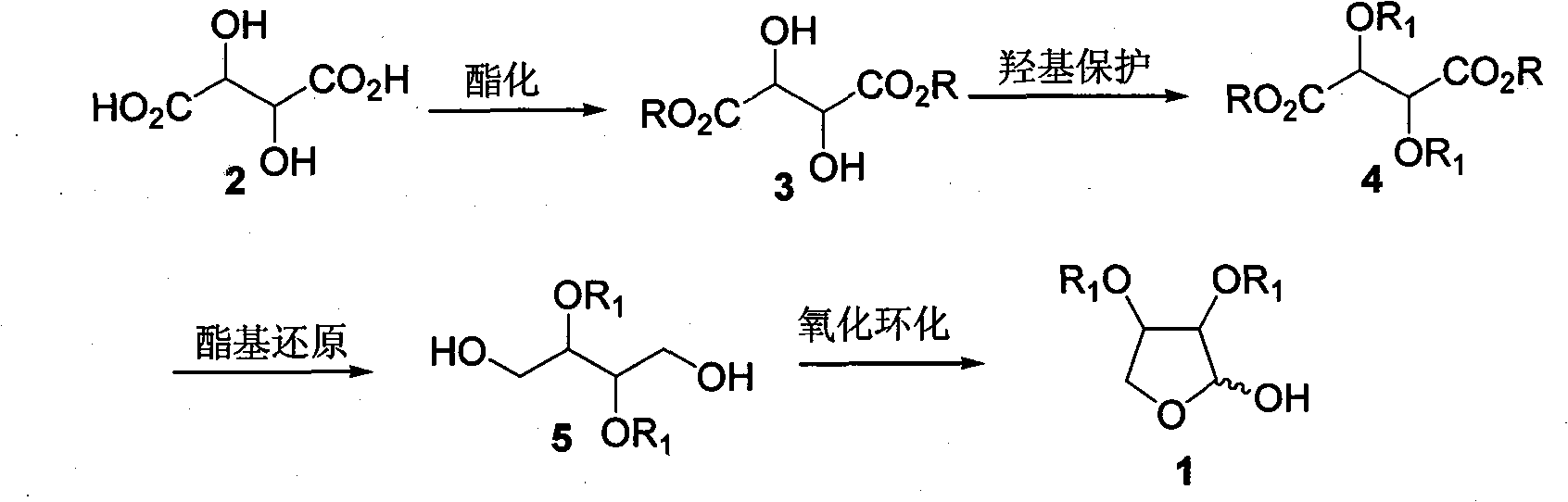
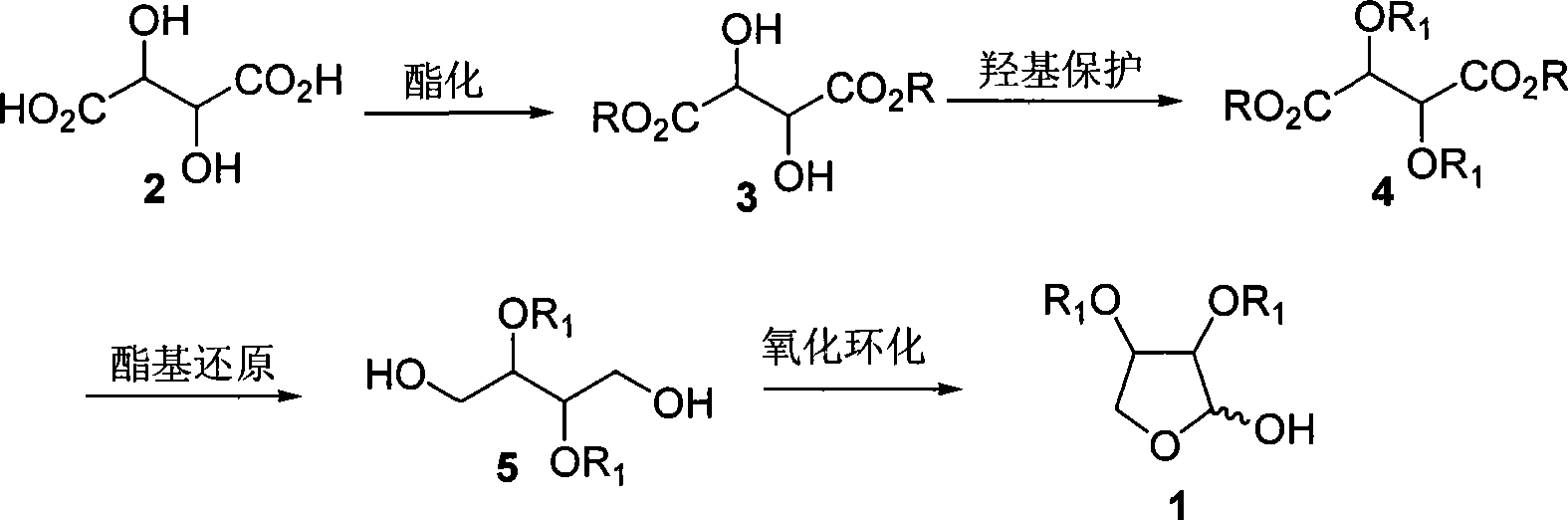
[0032] Step 1: Preparation of Diethyl L-tartrate [(2R,3R)-3, R=Et]
[0033] Dissolve L-tartaric acid (15g, 0.1mol) in 50ml of absolute ethanol, add 2ml of concentrated sulfuric acid dropwise, and stop the reaction after refluxing for 10h. Carefully add sodium bicarbonate powder until no gas escapes, filter with suction, and filter the filtrate with anhydrous sulfuric acid After drying over sodium and distilling off the solvent under reduced pressure, 18.5 g (90%) of diethyl L-tartrate was obtained as a pale yellow liquid, which was directly used in the next reaction without further purification. [α] D 20 =+8.7 (c=2.6, EtOH); IR(film): v max =3463, 2980, 1742cm -1 ; 1 H-NMR (400MHz, CDCl 3 ): δ=1.33(t, J=7.2Hz, 6H), 3.38(d, J=6.9Hz, 2H), 4.32(q, J=7.2Hz, 4H), 4.55(d, J=6.9Hz, 2H ); 13 C-NMR (100MHz, CDCl 3 ): δ=14.0, 62.4, 72.0, 171.5; MS (ESI) m / z (%): 229 (M+Na + , 100), 207 (M+H + ,2).
[0034] Step 2: Preparation of L-diethyl tartrate dibenzyl ether [(2R, 3R)-4, ...
Embodiment 2
[0041] Similar to Example 1, the difference is that in step one, anhydrous methanol is used to replace absolute ethanol and L-tartaric acid to react to obtain L-dimethyl tartrate [(2R, 3R)-3, R=Me]; in step two Substitute L-diethyl tartrate with L-dimethyl tartrate to obtain L-dimethyl tartrate dibenzyl ether [(2R, 3R)-4, R=Et, R 1 =Bn]; In step 3, replace diethyl tartrate dibenzyl ether with L-dimethyl tartrate dibenzyl ether to obtain (2R, 3R)-2,3-bis(benzyloxy)-1,4-butanedi Alcohol [(2R,3R)-5, R 1 =Bn].
Embodiment 3
[0043] Similar to Example 1, the difference is that in step one, anhydrous benzyl alcohol is used to replace absolute ethanol and L-tartaric acid to react to obtain L-dibenzyl tartrate [(2R, 3R)-3, R=Bn]; step two Use L-dibenzyl tartrate to replace L-diethyl tartrate to obtain L-dibenzyl tartrate dibenzyl ether [(2R, 3R)-4, R=R 1 =Bn]; Substitute diethyl tartrate dibenzyl ether with L-dibenzyl tartrate dibenzyl ether in step 3 to obtain (2R, 3R)-2,3-bis(benzyloxy)-1,4-butanedi Alcohol [(2R,3R)-5, R 1 =Bn].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com