Vinorelbine soft capsule and method for preparation and application thereof
A technology of vinorelbine and vinorelbine tartrate, which is applied in the field of capsule preparations and can solve problems such as complex prescriptions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The prescription screening of embodiment 1 soft capsule
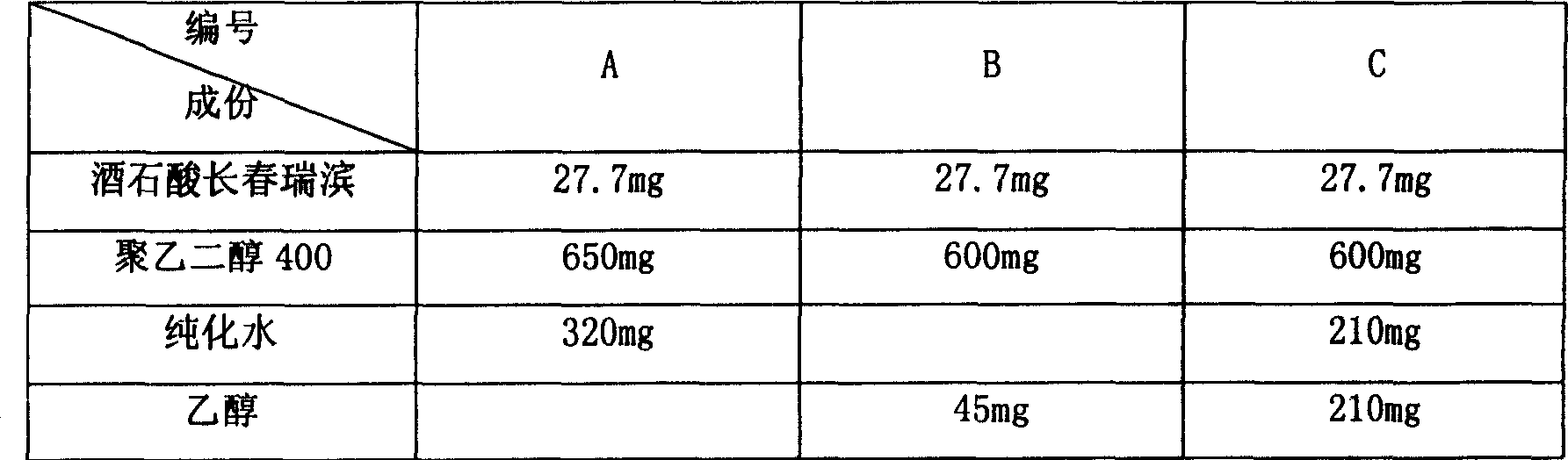
[0018] Preparation: According to the following prescriptions A-C, take the prescription amount of each auxiliary material in a clean container, add the prescribed amount of vinorelbine tartrate raw materials while stirring, stir to dissolve, and become a clear liquid. Then on the soft capsule machine, the liquid is encapsulated in the soft capsule material composed of gelatin and pressed into pellets, and dried.
[0019] The preparation composition of table 1 vinorelbine soft capsule
[0020]
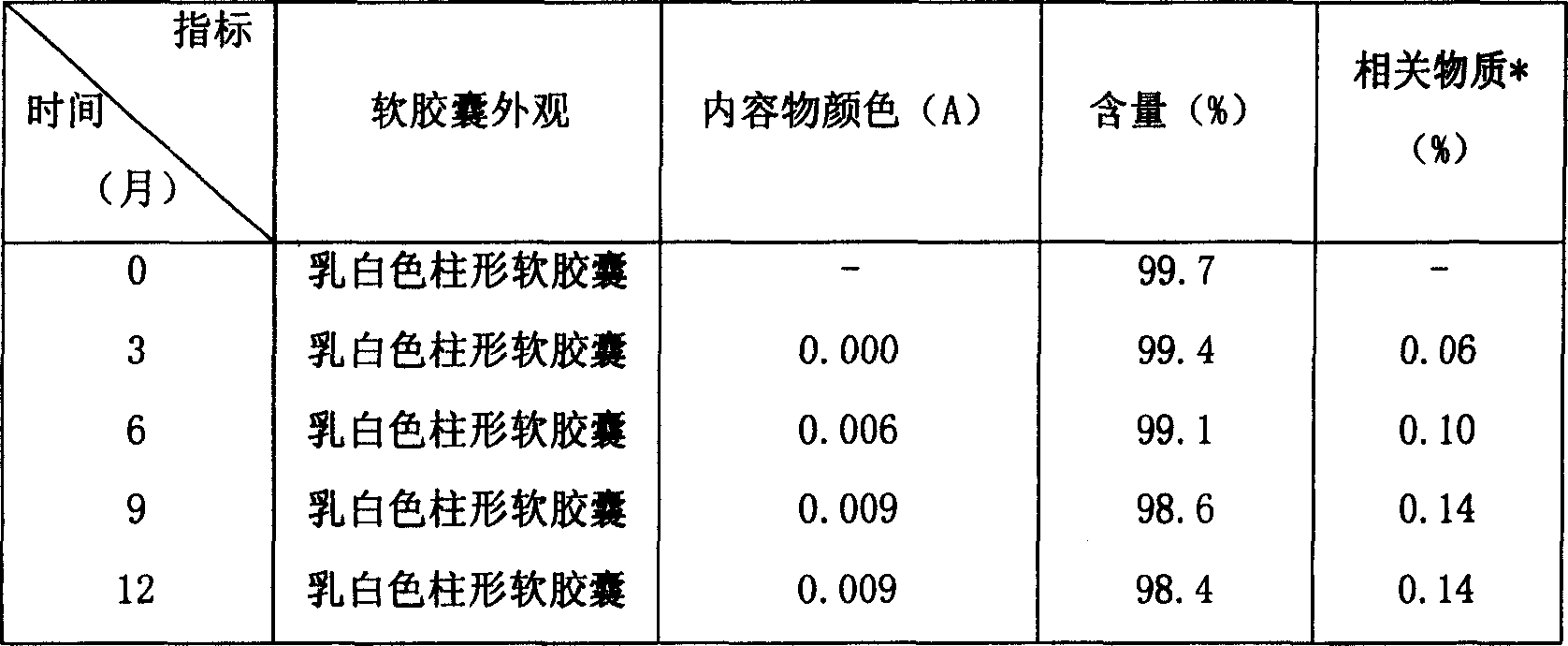
[0021] See Table 2 for the results of the stability investigation test of formulations A-C.
[0022] Table 2 Dissolution Data and Related Substances of Vinorelbine Soft Capsules
[0023] A
B
C
When the preparation is complete
Vinorelbine was completely dissolved,
Slightly sticky.
Vinorelbine was completely dissolved,
Slightly sticky.
Vinorelbine was completely...
Embodiment 2
[0026] Embodiment 2 soft capsule liquid filling composition prescription (according to 1000 grains):
[0027] Vinorelbine Tartrate 27.7g
[0028] Ethanol 70g
[0029] Macrogol 400 707.3g
Embodiment 3
[0030] Embodiment 3 soft capsule liquid filling composition prescription (according to 1000 grains):
[0031] Vinorelbine Tartrate 41.5g
[0032] Ethanol 65g
[0033] Macrogol 400 707.5g
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com