Process for producing polyketone
A manufacturing method and polyketone technology, applied in the field of polyketone manufacturing, can solve the problems of inability to obtain polymers and high-performance materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
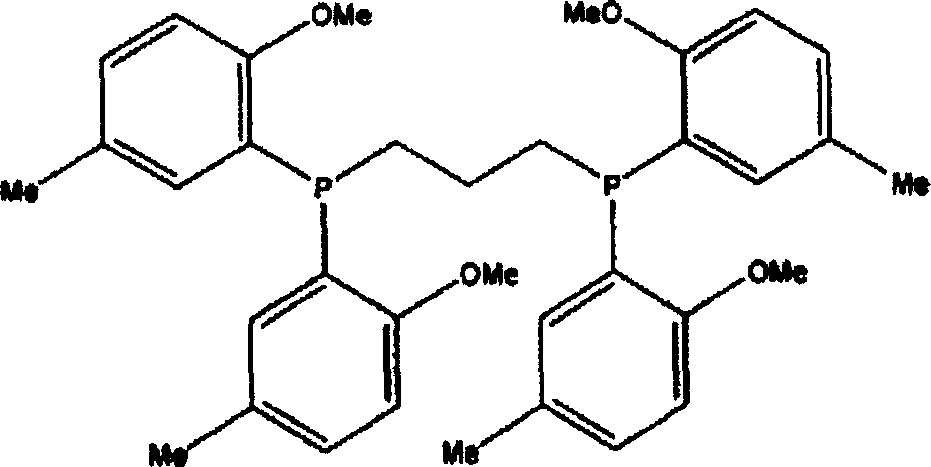
[0056] Dissolve 0.0140 g palladium acetate, 0.0398 g 1,3-bis[bis(2-methoxy-5-methylphenyl)phosphino]propane (BIBMAPP) and 0.0499 g trifluoroacetic acid with 0.4225 g benzothiazole In 100ml acetone. This solution was dissolved in a mixed solvent of 2497.5 ml of methanol and 1000 ppm of water, and the mixed solution was charged into a stainless steel autoclave which was replaced with nitrogen after deairing by vacuum. The autoclave was closed, then the contents were stirred at 800 rpm while heating. When the inner temperature reached 70° C., a mixed gas of carbon monoxide and ethylene in a molar ratio of 1:1.8 was added until the inner pressure of the autoclave was 100 bar. Keep the conditions of internal temperature 70° C. and internal pressure 100 bar, and keep stirring for 2 hours. After cooling, the gas in the autoclave was removed, and the contents were taken out. The reaction solution was filtered, washed several times with methanol, and then dried under reduced pressur...
Embodiment 2
[0060] Dissolve 0.0140 g of palladium acetate, 0.0398 g of 1,3-bis[bis(2-methoxy-5-methylphenyl)phosphino]propane and 0.0499 g of trifluoroacetic acid together with 0.4225 g of benzothiazole in 100 ml of acetone middle. This solution was dissolved in a mixed solvent of 2497.5 ml of methanol and 1000 ppm of water, and the mixed solution was charged into a stainless steel autoclave which was replaced with nitrogen after deairing by vacuum. The autoclave was closed, then the contents were stirred at 800 rpm while heating. When the inner temperature reached 80° C., a mixed gas of carbon monoxide and ethylene in a molar ratio of 1:2 was added until the inner pressure of the autoclave was 70 bar. Keep the conditions of internal temperature 80° C. and internal pressure 70 bar, and keep stirring for 2 hours. After cooling, the gas in the autoclave was removed, and the contents were taken out. The reaction solution was filtered, washed several times with methanol, and then dried und...
Embodiment 3
[0064]0.0140 g palladium acetate, 0.0398 g 1,3-bis[bis(2-methoxy-5-methylphenyl)phosphino]propane, 0.0249 g trifluoroacetic acid and 0.0215 g sulfuric acid were mixed with 0.4225 g benzothiazole Dissolve in 100ml acetone. This solution was dissolved in a mixed solvent of 2497.5 ml of methanol and 1000 ppm of water, and the mixed solution was charged into a stainless steel autoclave which was replaced with nitrogen after deairing by vacuum. The autoclave was closed, then the contents were stirred at 800 rpm while heating. When the inner temperature reached 70° C., a mixed gas of carbon monoxide and ethylene in a molar ratio of 1:1.8 was added until the inner pressure of the autoclave was 70 bar. Keep the conditions of internal temperature 70°C and internal pressure 70 bar, and keep stirring for 2 hours. After cooling, the gas in the autoclave was removed, and the contents were taken out. The reaction solution was filtered, washed several times with methanol, and then dried u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com