Method for the production of cyclohexanone
A technology of cyclohexanone and cyclohexanol, applied in the field of preparation of cyclohexanone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
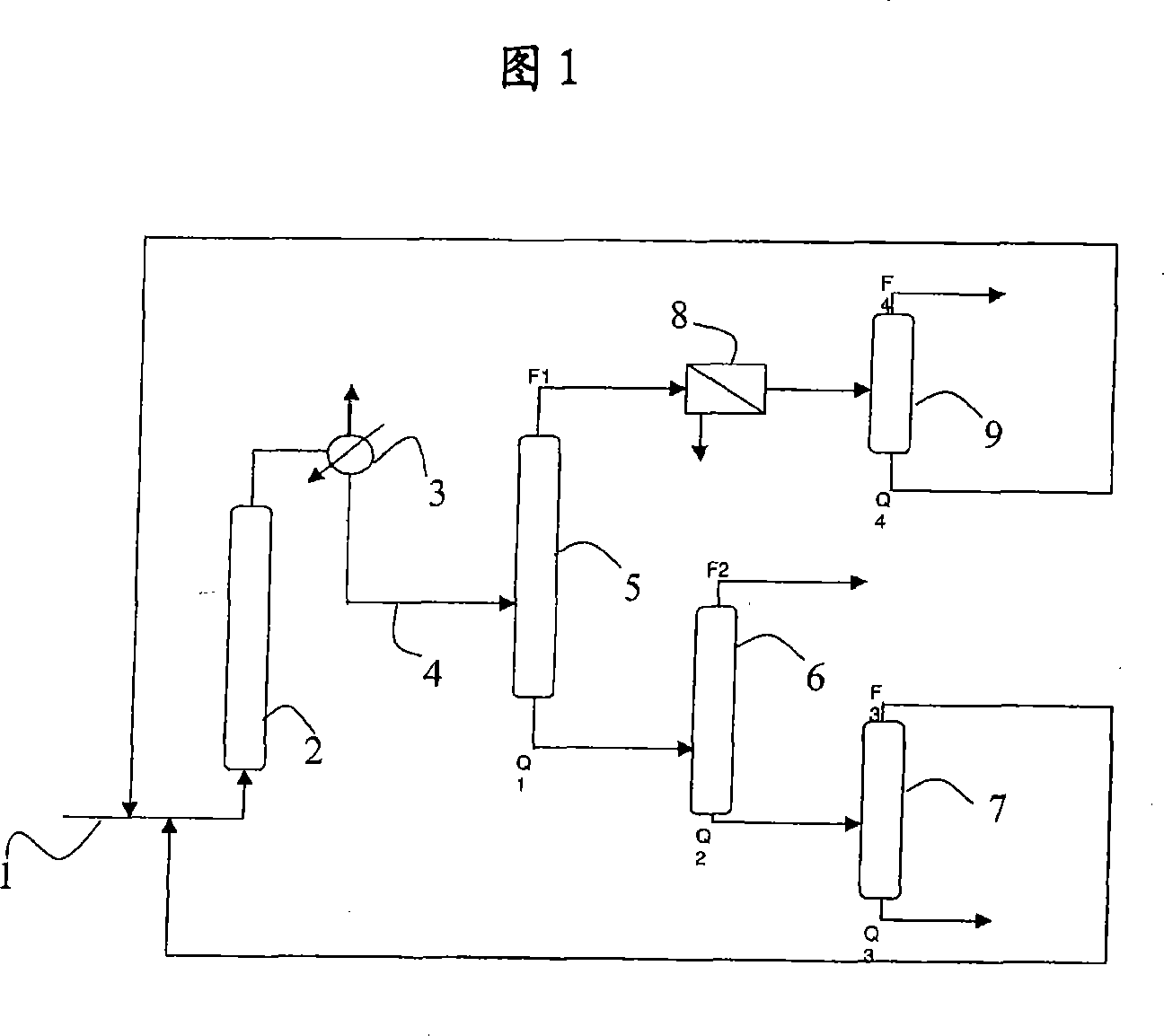
[0033] A cyclohexanol mixture containing 600 ppm cyclopentenal was fed at 1 into column reactor 2 .
[0034] Column 2 contains a fixed bed catalyst. The catalyst is copper oxide based.
[0035] The temperature in column 2 is 230°C. The conversion of cyclohexanol to cyclohexanone was 30%. The concentration of cyclopentenal in the reaction medium exiting reactor 2 is below the detection limit of known measuring methods, ie below 30 ppm.
Embodiment 2
[0037] Reactor 2 was charged with a mixture containing 59% by weight cyclohexanone, 39% by weight cyclohexanol, 0.5% by weight water and 1.5% by weight of heavy or light products considered to be impurities to be removed. As a particular impurity, cyclopentenal can be cited, and its concentration is 2950 ppm.
[0038] The rate at which this mixture was fed to Reactor 2 was 215 g / h.
[0039] The temperature of the reactor was 310°C.
[0040] The reaction medium flowing out of the reactor contained 80.6% by weight of cyclohexanone, 16.5% by weight of cyclohexanol and heavy or light impurities. The concentration of cyclopentenal in this medium was below the detection limit, ie below 30 ppm.
[0041] The conversion of cyclohexanol to cyclohexanone was 55%.
[0042] The reaction medium flowing from the reactor 2 is fed to a heat exchanger 3 and then to a first distillation column 5 through a line 4 .
[0043] This column comprises 22 theoretical stages and is carried out under ...
Embodiment 3
[0050] Example 2 was repeated, but a mixture containing 59 wt. .
[0051] The flow rate of this mixture into reactor 2 was 135 g / h.
[0052] The temperature of the reactor was 270°C.
[0053] The composition of the reaction medium flowing from reactor 2 is:
[0054] 75.2% by weight cyclohexanone, 22.3% by weight cyclohexanol and heavy or light impurities. The concentration of cyclopentenal in this medium was below the detection limit, ie below 30 ppm.
[0055] The conversion of cyclohexanol to cyclohexanone was 44%.
[0056] as fraction F 2 The cyclopentenal content in the recovered cyclohexanone is lower than 30 mg / kg, and the transmittance in the UV test at 230 nm is 89.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com