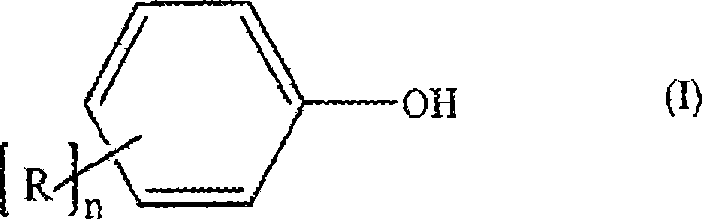
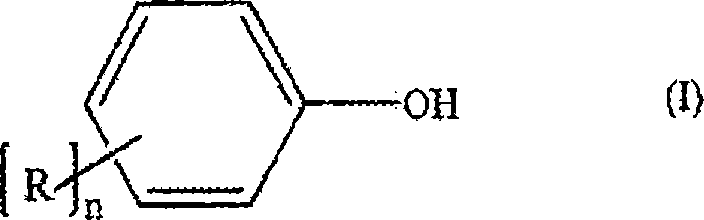
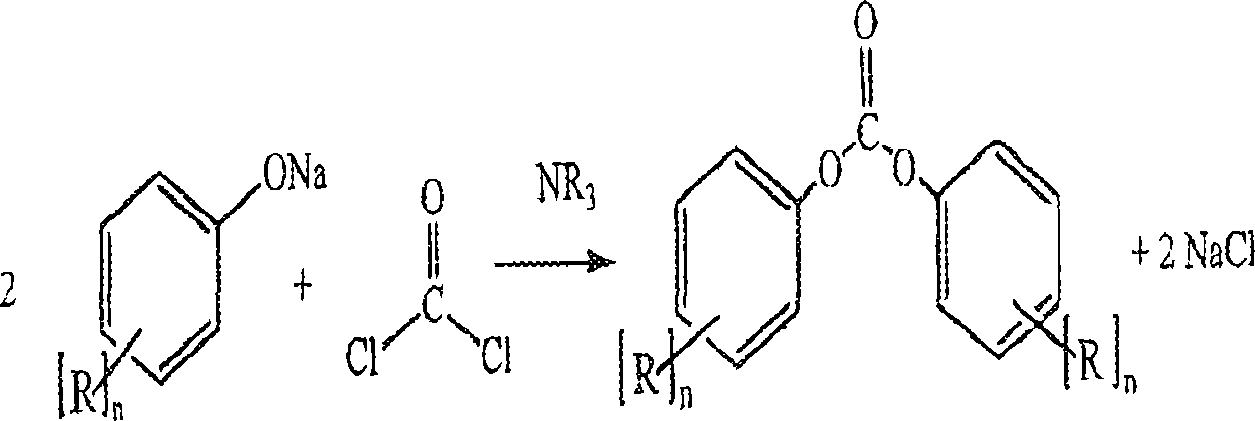
Process for the production of diaryl carbonates and treatment of alkalichloride solutions resulting therefrom
A technology of diaryl carbonate and alkali metal chloride, which is applied in the field of joint process, and can solve the problems of high cost and large amount of energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Addition of sodium chloride-containing reaction waste water to sodium chloride electrolysis—Addition of 17% (weight) sodium chloride solution from DPC manufacture.
[0083] A mixture of 14.5% (weight) sodium hydroxide solution of 145.2kg / h and 48.3kg / h of phenol with 86.2kg / h of methylene chloride and 27.5kg / h of carbonyl chloride (based on phenol exceeding 8 % (mol))) were mixed together in a vertically placed and cooled tubular reactor, the reaction mixture was cooled to 33° C., and the pH was measured to be 11.5 after an average residence time of 15 seconds. In the second step of the process, 5.4 kg / h of 50% NaOH were subsequently metered into the reaction mixture so that the pH value of the second reaction stage was 8.5 after a further 5 minutes of dwell. In the continuously running reactions, existing metering fluctuations were compensated by adjusting the NaOH added in each case. In the second step of the process, the reaction mixture is continuously m...
Embodiment 2
[0088] Example 2: Addition of reaction waste water containing sodium chloride to sodium chloride electrolysis using a gas diffusion electrode - addition of a 17% by weight sodium chloride solution from DPC manufacture.
[0089] The composition of the waste water is the same as the composition of the waste water obtained according to Example 1. Since hydrogen is not required for the manufacture of DPC, the generation of hydrogen can be omitted during the electrolysis process. Electrolysis is therefore carried out with gas diffusion electrodes. The current density is 4kA / m 2 , the outlet temperature on the cathode side is 88°C, and the outlet temperature on the anode side is 89°C. Electrolyzers with standard anodic coatings from DENORA (Germany) were used. A Nation 982 WX ion exchange membrane from DuPont was used. The electrolytic voltage is 2.11 volts. The sodium chloride concentration of the solution removed from the anode chamber was 17% by weight NaCl. 0.166 kg / h of 1...
Embodiment 3
[0092] Example 3: Addition of sodium chloride-containing reaction waste water to sodium chloride electrolysis with gas diffusion electrodes - addition of 17% by weight sodium chloride solution (reaction waste water) from DPC production.
[0093] The composition of the waste water is the same as the composition of the waste water obtained according to Example 1. Since hydrogen is not required for the manufacture of DPC, the generation of hydrogen can be omitted during the electrolysis process. Therefore electrolysis is carried out with a gas diffusion electrode at a current density of 4kA / m 2, the outlet temperature on the cathode side is 88°C, and the outlet temperature on the anode side is 89°C. An electrolytic cell with standard anodic coating from DENORA (Germany) was used. DuPont's Nation 2030 WX ion exchange membrane was used. The electrolytic voltage is 1.96 volts. The sodium chloride concentration of the solution removed from the anode chamber was 15% by weight NaCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com