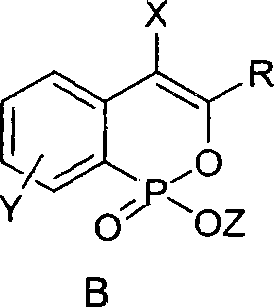
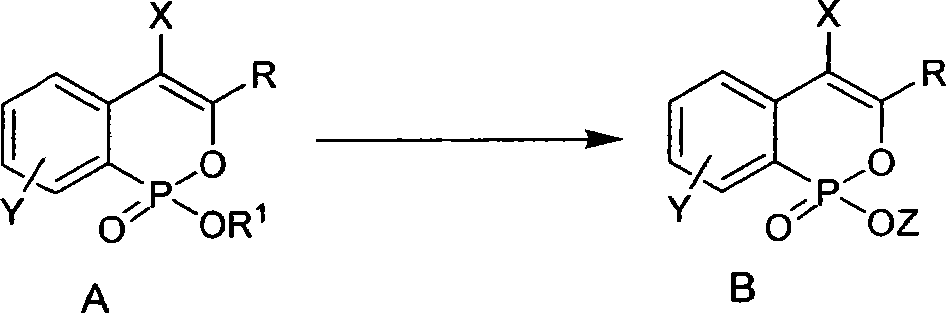
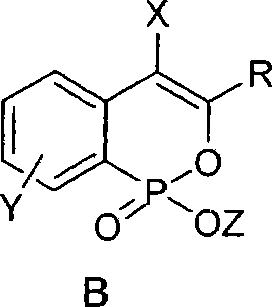
Phosphine isocoumarin salt and preparation method thereof
A technology of isocoumarin and alkali metal salt, which is applied in the field of phosphine isocoumarin salt and its preparation, can solve the problems of limited practical application and poor water solubility, and achieve good application prospects, improved water solubility, and simple and convenient preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] Typical operation for preparing phosphineisocoumarin alkali metal salt: under nitrogen protection, add 50mg (0.175mmol) 3-phenylphosphineisocoumarin ethyl ester, 9mg (0.210mmol) LiCl and 1ml anhydrous acetone into a 25ml egg-shaped bottle , reflux (oil bath temperature about 60° C.) and stirred for 12 hours. TLC detected that the reaction was complete. The acetone solvent was removed under reduced pressure, and the obtained white solid was washed with icy acetone. After drying, 37 mg of white solid was obtained, and the yield was 80%. Its solubility in water is about 0.080g / 1g water. 1 H NMR: δ7.74-7.83 (m, 3H), 7.22-7.34 (m, 6H), 6.82 (s, 1H); ESI-MS: 287 (M+Na, 100).
Embodiment 2
[0022]
[0023] The typical operation of preparing phosphine isocoumarin sodium salt: wherein the phosphine isocoumarin ester is 7-chloro-3-phenylphosphine isocoumarin ethyl ester, the molar amount of the reactant is the same as in Example 1, and the experimental operation is basically the same as the implementation Example 1, the difference is that the alkali metal salt used is NaI, the reaction solvent is anhydrous DMF, the reaction temperature is 80° C., and the reaction is carried out for 15 hours. After the reaction, toluene is added to azeotropically remove the DMF solvent under reduced pressure. The product was a white solid in 82% yield. Its solubility in water is about 0.085g / 1g water. 1 H NMR: δ7.74-7.80(m, 2H), 7.66-7.75(m, 1H), 7.29-7.38(m, 4H), 6.75(s, 1H); ESI-MS: 337(M+Na, 100 ).
Embodiment 3
[0025]
[0026] The typical operation of preparing phosphine isocoumarin potassium salt: wherein the phosphine isocoumarin ester used is 7-methoxy-4-bromo-3-phenylphosphine isocoumarin ethyl ester, and the molar amount of the reactant is the same as in Example 1 , the experimental operation is basically the same as in Example 2, except that the alkali metal salt used is KI, the reaction solvent is anhydrous 1,4-dioxane, the reaction temperature is 80°C, and the reaction is carried out for 12 hours. After the reaction, the reaction is removed under reduced pressure. 1,4-Dioxane solvent. The product was a white solid in 75% yield. Its solubility in water is about 0.075g / 1g water. 1 H NMR: δ7.81-7.86 (m, 1H), 7.61-7.65 (m, 2H), 7.38-7.42 (m, 3H), 7.21-7.32 (m, 2H), 3.85 (s, 3H); ESI- MS: 427 (M+Na, 100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com