Antiangiogenic agents and aldesleukin
A kind of aldesleukin and anti-vascular technology, applied in cardiovascular system diseases, anti-tumor drugs, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
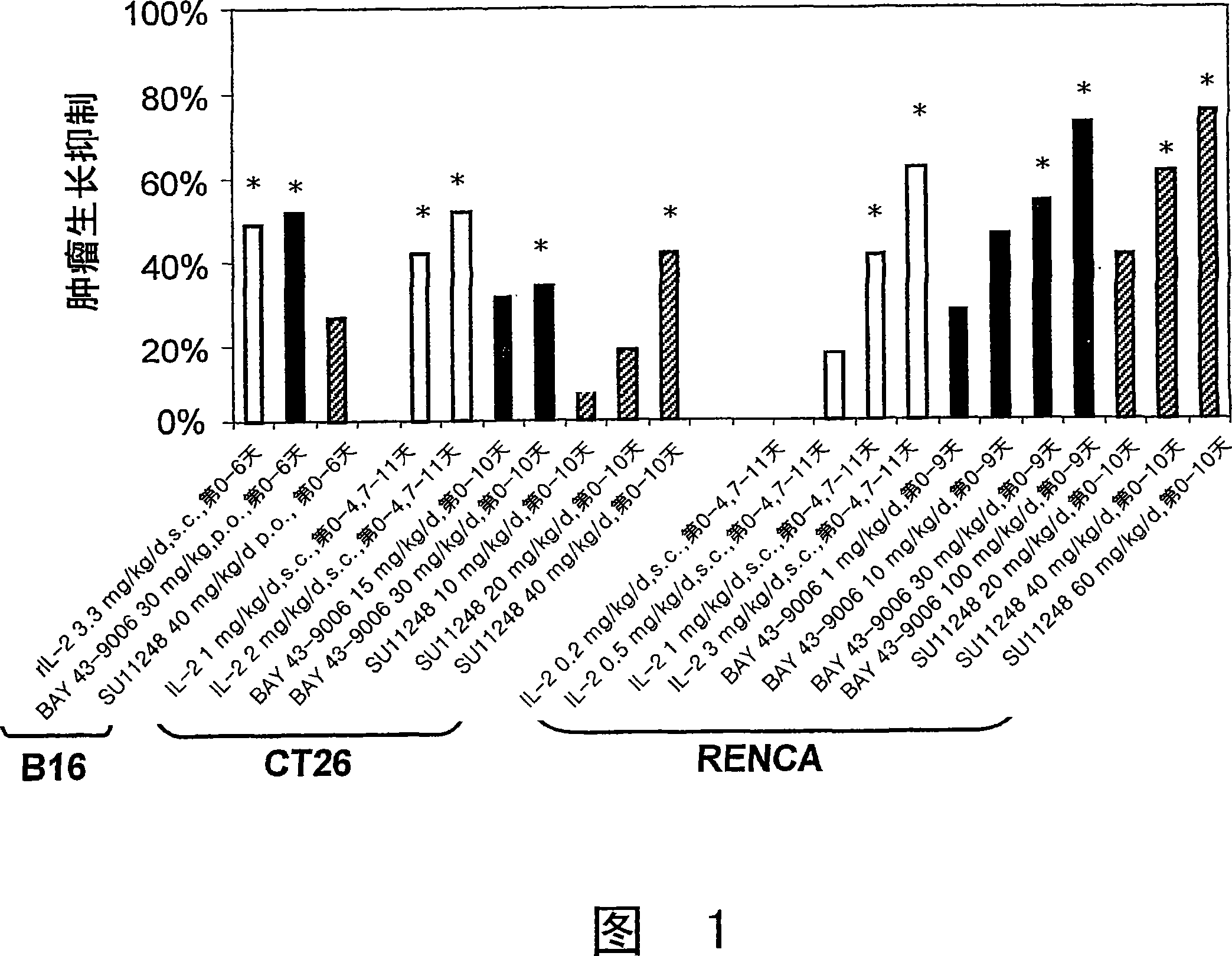
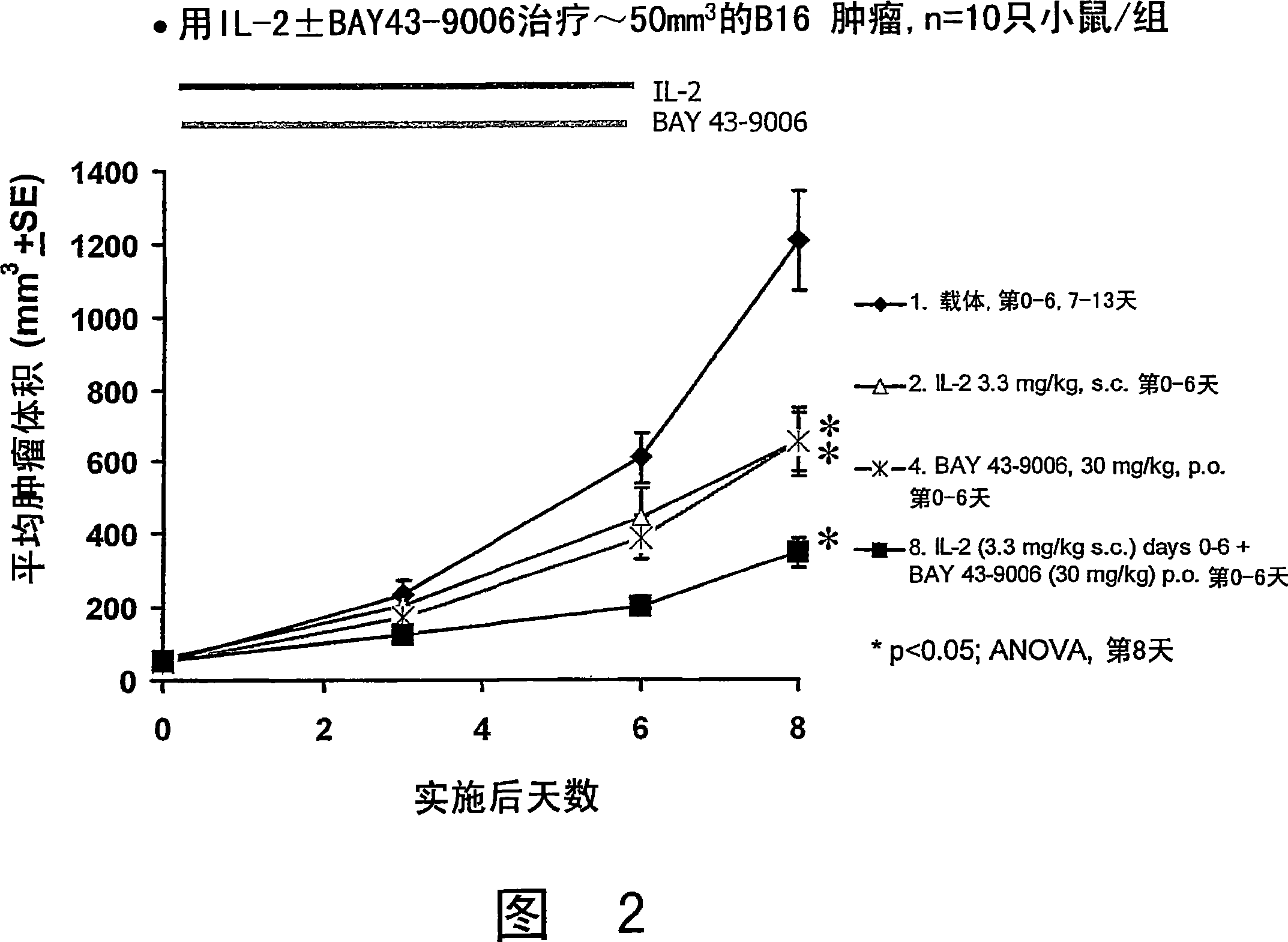
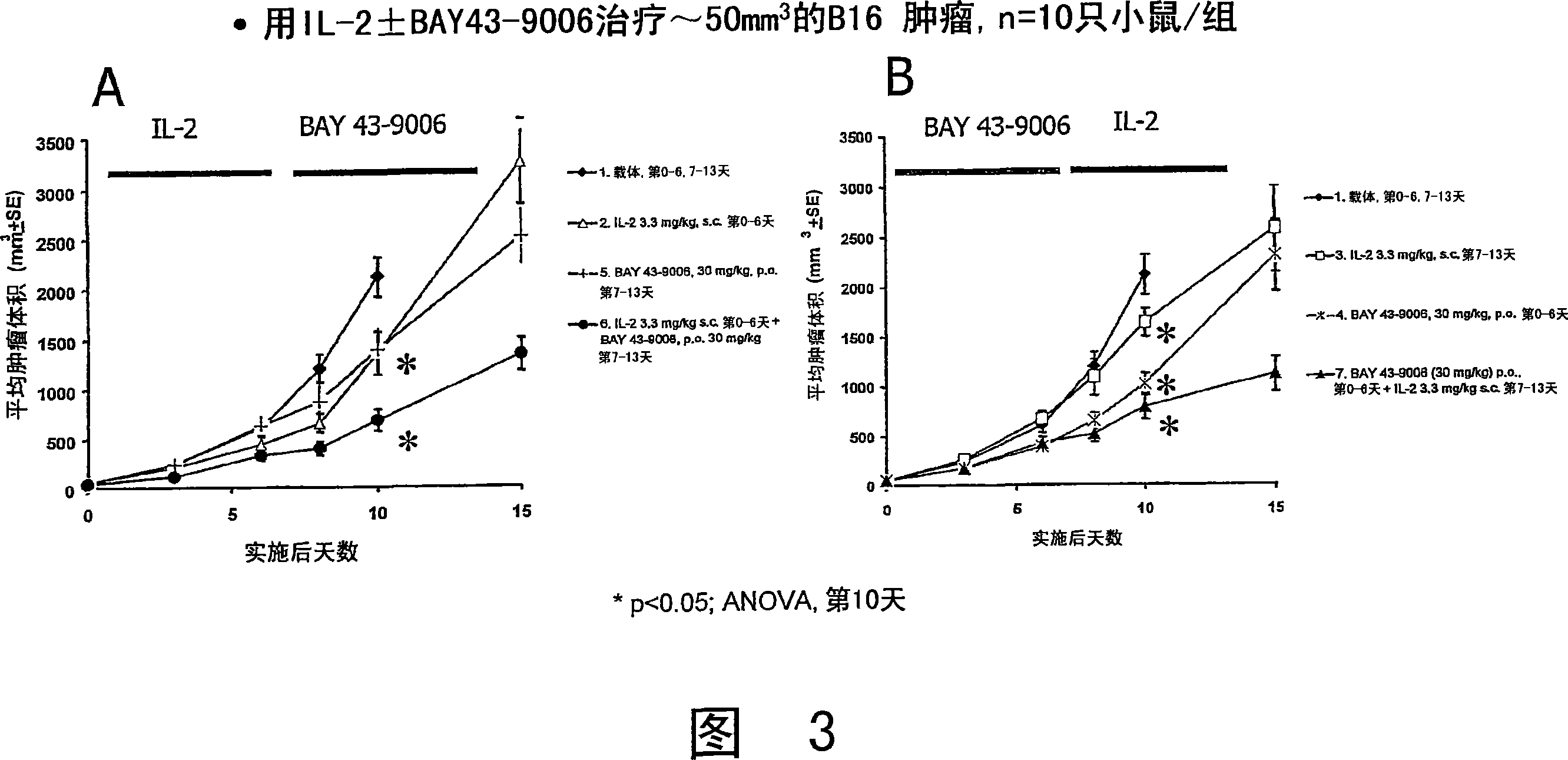
Image
Examples
Embodiment 1
[0332] Compositions co-administered with rIL-2
[0333] A. Compound
[0334] Table 1: Quinazoline Examples
[0335]
[0336] Table 2: Indolinone compounds
[0337]
[0338] Table 3: Other compounds
[0339]
[0340]
[0341] Table 4: Macrocyclic compounds
[0342]
[0343] Table 5: Isoindoline compounds
[0344]
[0345] B. Synthesis
[0346] Quinazoline
[0347] Case 1:
[0348]
[0349]Scheme 1 describes a modular approach to the synthesis of multiple substituted quinazoline compounds. AG refers to an activating group such as halogen, triflate or ketone, in which up to 4 AG groups may be present. Since the 4-chloro position shown in the third step is more active than positions 5-8 on the benzyl ring, the substitution of NHRR' is unlikely to simultaneously replace AG at any position 5-8. Subsequently, the last step of substituting R" for the AG group can be carried out in the presence of a weak-to-moderate base. Alternatively, the AG group can be modified to produce...
Embodiment 2
[0373] Preparation of rIL-2
[0374] Aldesleukin, rIL-2 (NSC3773364) (Proleukin _ , Chiron): The messenger RNA of the human Jurkat cell line was used to produce double-stranded cDNA, which was hybridized to the pBR322 plasmid. Use the one corresponding to the short IL-2 basic sequence 32 The p-labeled oligonucleotide probe identifies clones containing the IL-2 gene. Insert the gene into a region of the pBR322 plasmid with convenient restriction sites. Insert a suitable promoter and ribosome binding site before the IL-2 gene, and the resulting expression clone encodes a modified recombinant rIL-2. Cysteine at position 125 was conservatively substituted with serine by cloning IL-2 in vitro mutagenesis. The obtained molecule is indistinguishable from natural IL-2 in terms of in vitro biological activity. Culture the E. coli production strain carrying the aldesleukin gene in the fermentor. The culture was harvested, and aldesleukin was extracted. A series of chromatography steps are...
Embodiment 3
[0378] Comparison of treatment with rIL-2 composition alone and combined treatment with rIL-2 composition and anti-angiogenesis agent Table 6 summarizes the results of high-dose rIL-2.
[0379] Table 6: Clinical trials of a single drug rIL-2 bolus injection program 1
[0380] Author
Scheme rIL-2
Dose range
(MIU)
Number of patients
CR
PR
Median duration of response
/ Median survival rate (mos)
Atkins and others (7)
Q8 hours 1-5 days
24 / m 2
71
4
8
16+ / 15.5
Fyfe et al (8)
Q8 hours 1-5 days
0.6-0.72 / kg
255
12
24
20.3 / 16.3
Yang et al (9)
Q8 hours 1-5 days
0.72 / kg
65
2
11
NS
Rosenberg et al (10)
Q8 hours 1-5 days
Q8 hours 1-5 days
0.072 / kg
0.72 / kg
60
149
4
10
5
20
NS
15 / 20
Rosenberg et al (11)
Q8 hours 1-5 days
0.72 / kg
48
4
6
NS
Taneja and others (12)
Q8 hours 1-5 days
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com