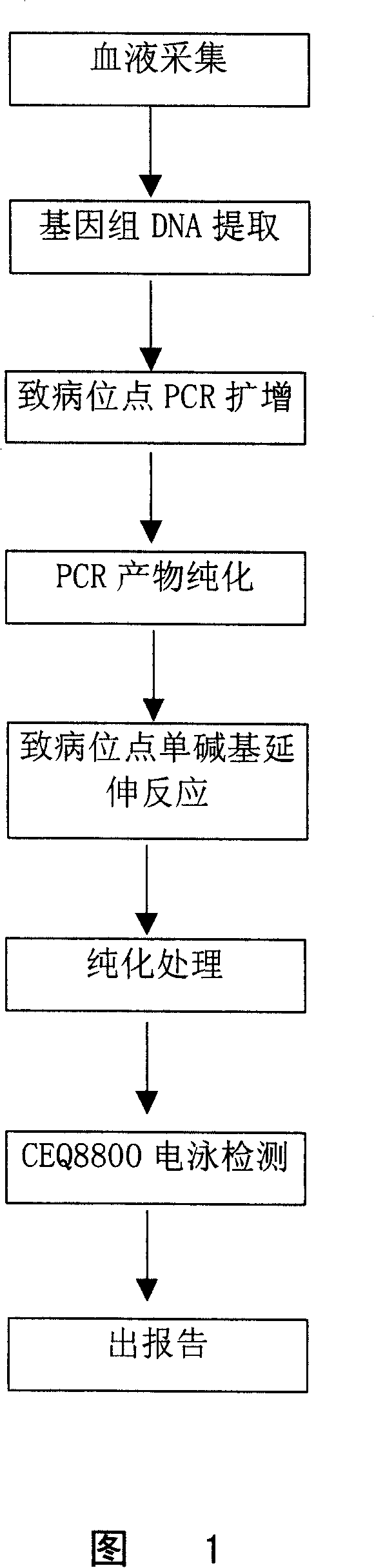
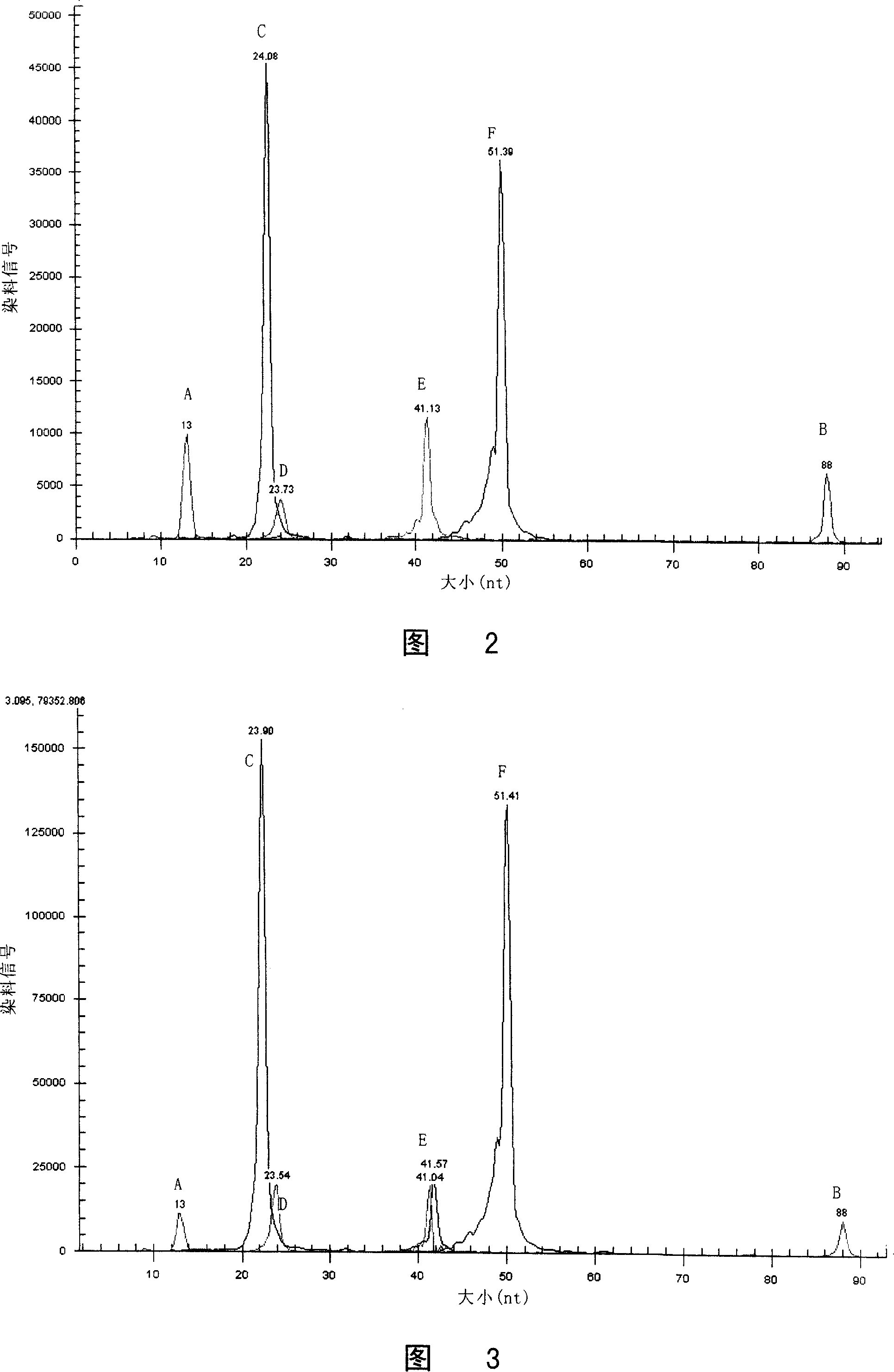
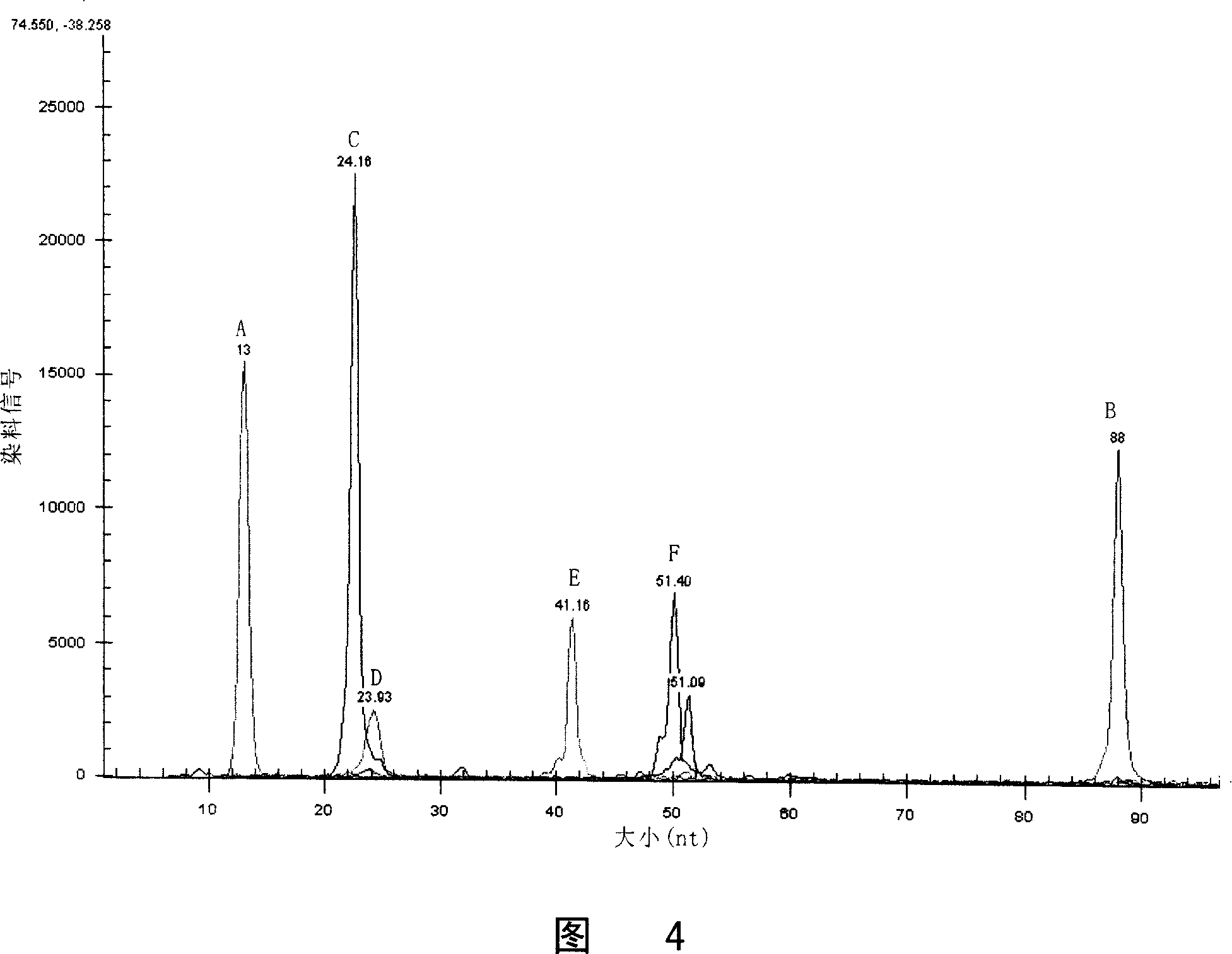
Method for detecting multiple endocrine adenoma II gene mutation
A gene and mutation technology, applied in biological testing, material inspection products, etc., can solve the problems of low incidence of MEN2, different RET gene mutation sites, and no RET gene mutation status
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] The RET gene mutation research of embodiment 1MEN2A
[0113] 1. Object
[0114] A total of 15 MEN2A proband cases were collected from January 2002 to February 2006. These cases came from different regions, including: Shanghai, Zhejiang, Jiangsu, Fujian, Guangdong, Jiangxi, Shandong, Shaanxi, Sichuan, Liaoning, etc. . The family members of each proband were investigated for clinical history, blood biochemistry and DNA specimens were collected. A total of 119 samples were collected.
[0115] 2 RET proto-oncogene detection in the proband and family members
[0116] 2.1 Extraction of peripheral blood genomic DNA (phenol-chloroform extraction method).
[0117] 5ml of whole blood from the median forearm vein of the proband and family members of 119 people was collected, placed in a 15ml centrifuge tube containing 1ml of ACD anticoagulant, and turned upside down several times to fully mix the blood and anticoagulant. Specific steps:
[0118] (1) Add 8 ml of ice-precooled...
Embodiment 2
[0166] The RET gene mutation research of embodiment 2MEN2B
[0167] 1. Object
[0168] From April 2002 to September 2005, 5 MEN2B families were collected from all over the country, including 5 probands and 23 family members.
[0169] 2RET proto-oncogene detection
[0170] 2.1 Extraction of genomic DNA from peripheral blood
[0171] Collect 5ml of peripheral anticoagulant blood from the proband and family members, and use the U-gene Blood DNA Kit to extract the blood genomic DNA. The specific steps are:
[0172] (1) Add 250ul whole blood sample into a 1.5ml centrifuge tube.
[0173] (2) Add 250ul of XY buffer solution (U-gene), shake and mix well.
[0174] (3) Add 20 ul of proteinase K (10 mg / ml), shake vigorously and mix well.
[0175] (4) Incubate at 56°C overnight, and the color will turn dark green.
[0176] (5) Add 260 μl of absolute ethanol, shake to mix, centrifuge at 12,000 rpm for 1 minute, and shake the solid precipitate in the solution to the bottom of the tube...
Embodiment 3
[0193] Embodiment 3 detects the method for RET gene mutation
[0194] Since it is very cumbersome to carry out the RET gene mutation by taking the steps described in the foregoing Example 1 or Example 2, especially sequencing is required. The disadvantage of sequencing is that it takes a long time (about 3 days) and the process is complicated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com