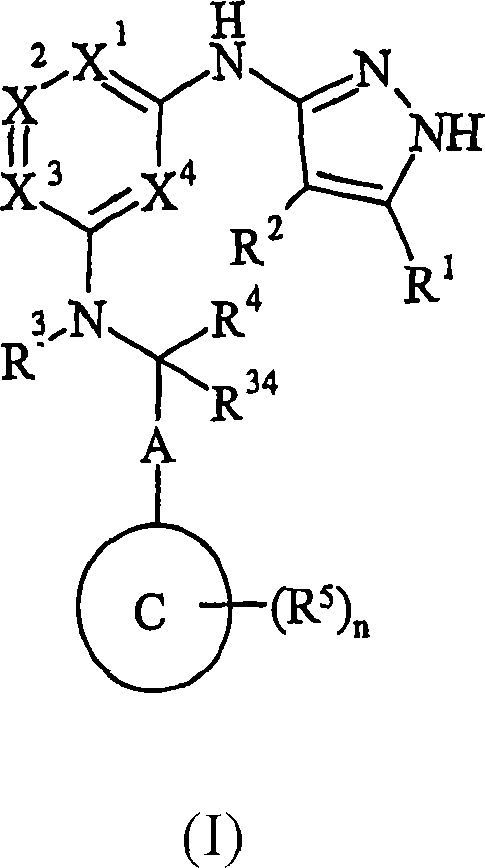
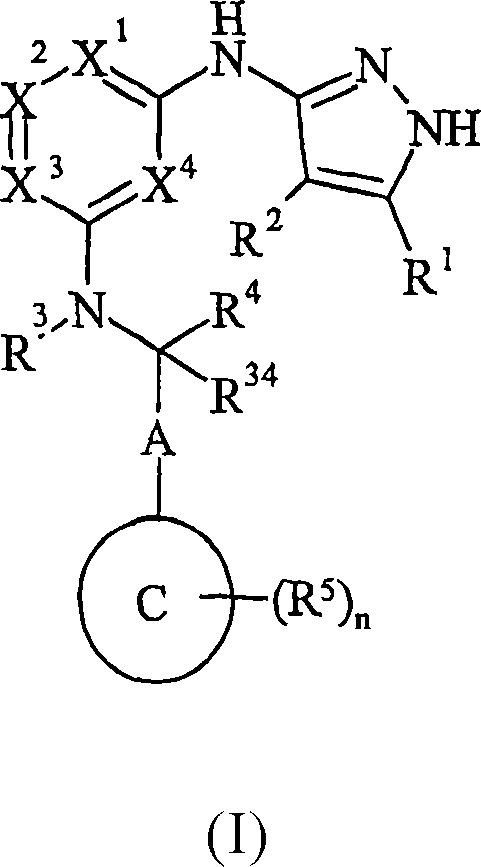
Pyrazolylaminopyridine derivatives useful as kinase inhibitors
A kind of amino, carbamoyl technology, applied in the field of novel pyrazole derivatives, can solve problems such as Trk expression increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0463] (S)-6-(5-cyclopropyl-1H-pyrazol-3-ylamino)-5-fluoro-2-(1-(4-fluorophenyl)ethylamino) Nicotinonitrile
[0464] In a sealed test tube, combine 2-chloro-6-(5-cyclopropyl-1H-pyrazol-3-ylamino)-5-fluoronicotinonitrile (Method 1; 0.8g, 2.8mmol) and (S) -1-(4-Fluorophenyl)ethanamine (0.8 g, 5.6 mmol) was added in one portion to a solution of n-BuOH (4 ml) and DIEA (0.5 g, 3.7 mmol). The reaction was heated to 140°C for 48 hours, then cooled to 25°C and concentrated. The obtained residue was purified by column chromatography (DCM-MeOH=50:1) to obtain the title compound (0.55 g, 50%). 1 H NMR (400MHz, CDCl 3)δ8.44 (br s, 1H), 7.37-7.33 (m, 2H), 7.27 (d, J=9.6Hz, 1H), 7.07-7.03 (m, 2H), 6.11 (s, 1H), 5.24- 5.20 (m, 2H), 1.87-1.83 (m, 1H), 1.60 (d, J=6.2Hz, 3H), 1.01-0.98 (m, 2H), 0.79-0.65 (m, 2H). MS: Calculated: 380; Found: [M+H] + 381.
[0465] (S)-6-(5-cyclopropyl-1H-pyrazol-3-ylamino)-5-fluoro-2-(1-(4-fluorophenyl)ethylamino) Nicotinamide
[0466] At 25°C, t...
Embodiment 3
[0468] (S)-3-(Aminomethyl)-N 6 -(5-cyclopropyl-1H-pyrazol-3-yl)-5-fluoro-N 2 -(1-(4-fluorobenzene Base) ethyl) pyridine-2,6-diamine
[0469] To MeOH solution (5ml) was added (S)-6-(5-cyclopropyl-1H-pyrazol-3-ylamino)-5-fluoro-2-(1-(4-fluorophenyl)ethyl Amino)nicotinonitrile (Example 1; 0.15 g, 0.4 mmol), conc. HCl (0.1 ml) and Pd (10% by weight dry on activated carbon, 0.12 g). Then, the mixture was washed with N 2 Flood, empty, then place it at 40psi H 2 Set aside for 6 hours. Then, the reaction was evacuated with N 2 Flooded, filtered, washed with MeOH (3 x 30ml) and concentrated. The resulting solid was dissolved in a mixture of DCM-MeOH (50:1, 100ml), to which was added Na 2 CO 3 Saturated aqueous solution (100ml) and the mixture was shaken vigorously for 30 minutes. Then, the layers were separated and the aqueous layer was extracted with DCM (3 x 100ml). The combined organic layers were dried, filtered and concentrated. The obtained solid was purifi...
Embodiment 4
[0471] (S)-N-((6-(5-cyclopropyl-1H-pyrazol-3-ylamino)-5-fluoro-2-(1-(4-fluorophenyl)ethyl Amino)pyridin-3-yl)methyl)acetamide
[0472] To a THF-DCM (1:1, 3ml) mixture in a round bottom flask at 0°C was placed (S)-3-(aminomethyl)-N 6 -(5-cyclopropyl-1H-pyrazol-3-yl)-5-fluoro-N 2 -(1-(4-fluorophenyl)ethyl)pyridine-2,6-diamine (Example 3; 0.08g, 0.2mmol) and TFP resin loaded with acetic acid (1.4mmol / g loading, 0.2mmol ). The resulting solution was shaken vigorously at 0°C for 45 minutes and filtered. The obtained resin was washed with THF-DCM solution (1:1, 3×5 ml, 30 minutes each time). The resulting organic layers were combined and concentrated. The resulting solid was purified by reverse phase column chromatography (5-50% 2 CH in O 3 CN, over 400ml) to give the title compound (0.045g, 50%). 1 H NMR (400MHz, CD 3 OD) δ7.34-7.33(m, 2H), 7.11(d, J=10.7Hz, 1H), 7.01-6.97(m, 2H), 5.14-5.04(m, 1H), 4.30-4.17(m, 2H ), 1.99 (s, 3H), 1.88-1.81 (m, 1H), 1.50 (d, J=6.8Hz, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com