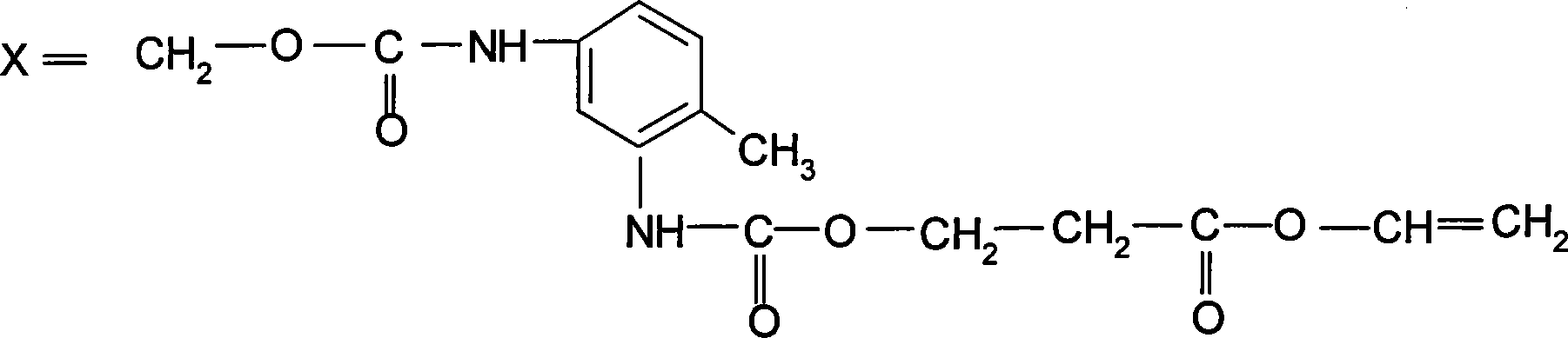
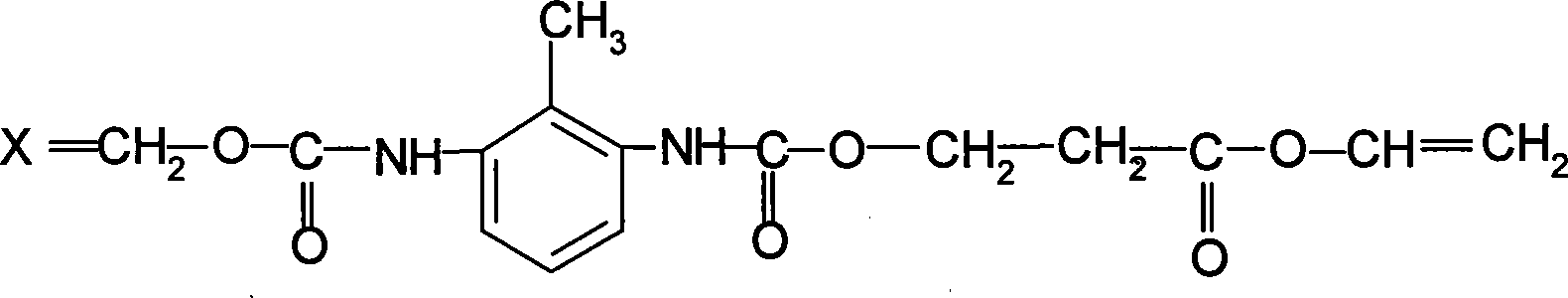
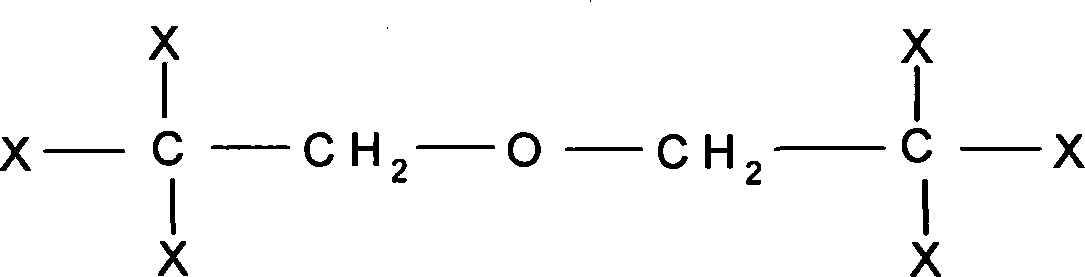Acrylic polyurethane having star-structure six functional groups and synthesizing method thereof
A technology of acrylic polyurethane and star structure, applied in the field of acrylic polyurethane and its synthesis, to achieve the effects of excellent mechanical properties, fast curing speed and large shielding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In a 1000ml stirred three-neck flask, 50g of dipentaerythritol, 203.8g of toluene-2,4 diisocyanate (TDI) and 0.076g of catalyst dibutyltin dilaurate were added. Control the temperature and react at 18°C for 100 minutes, take a sample to determine the isohydrogen content of 19.4025% (mass concentration), add 151.8g of hydroxyethyl acrylate, and add 1.214g of polymerization inhibitor p-hydroxyanisole. Control the temperature and react at 22° C. for 5 hours, then take a sample to measure the isohydrogen content of 0.1643% (mass concentration), stop the reaction, and obtain 406.9 g of the product, which is colorless, clear and transparent.
Embodiment 2
[0038] In a 3000ml stirred three-neck flask, 235g of dipentaerythritol, 965.7g of toluene-2,4 diisocyanate (TDI) and 0.262g of catalyst dibutyltin dilaurate were added. The temperature was controlled at 25°C for 90 minutes, and the isohydrogen content was determined to be 19.5276% (mass concentration) by sampling. 654 g of hydroxyethyl acrylate was added, and 4.578 g of polymerization inhibitor 2,6-di-tert-butyl-p-cresol was added simultaneously. After controlling the temperature to react at 31° C. for 4 hours, sampling and determination of the isohydrogen content was 0.1342% (mass concentration), and the reaction was stopped to obtain 1.859 kg of the product, which was colorless, clear and transparent.
Embodiment 3
[0040] In a 20L pilot scale reactor, 1.5kg of dipentaerythritol, 6.17kg of toluene-2,4 diisocyanate (TDI) and 1.27g of catalyst dibutyltin dilaurate were added. The temperature was controlled at 37° C. for 80 minutes, and the isohydrogen content was determined to be 19.4852% (mass concentration) by sampling. 4.22 kg of hydroxyethyl acrylate was added, and 25.3 g of a polymerization inhibitor triphenylphosphine was added at the same time. After controlling the temperature to react at 43°C for 3.5 hours, sampling and determination of the isohydrogen content was 0.1275% (mass concentration), and the reaction was stopped to obtain 11.917 kg of the product, which was colorless, clear and transparent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com