Adenine bisphosphonate and preparation method thereof and application in pharmaceutical preparations
A bisphosphonate and adenine technology, applied in the field of adenine bisphosphonate and its preparation and application in pharmaceutical preparations, to achieve long-lasting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
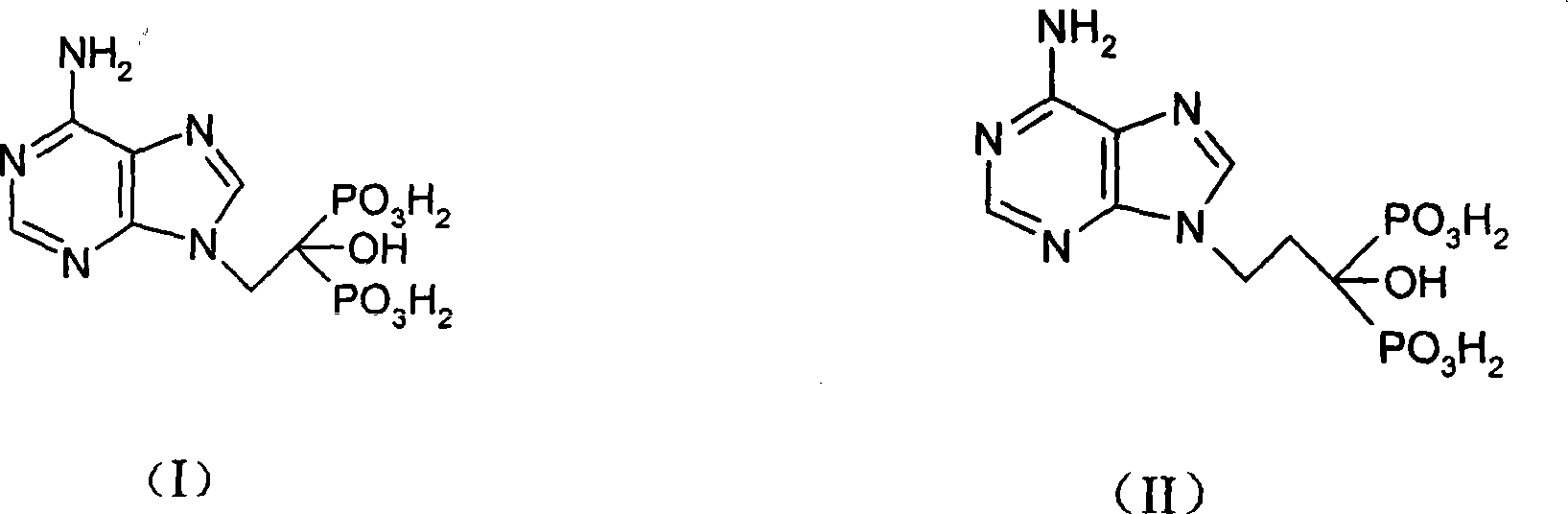
[0114] Example 1 Synthesis of compound I of the present invention
[0115] (a) Synthesis of (6-amino-purin-9-yl)-ethyl acetate:
[0116] Put adenine (10.8g, 0.08mol) in a four-neck flask, add 100ml of DMF, heat to 100°C, adenine is insoluble, after cooling down to room temperature, add anhydrous Na 2 CO 3 (8.2g, 0.08mol), reacted at 100°C for 30min, lowered to 80°C, and slowly added ethyl monochloroacetate (17ml, 0.16mol) dropwise. After dropping, keep the temperature at 80° C. and stir the reaction. TLC monitors the reaction process. After 8 hours, after cooling, filter with suction to obtain a dark yellow solid. After drying with an infrared lamp, the total amount of solid is 19 g.
[0117] Purification of (6-amino-purin-9-yl)-ethyl acetate
[0118] Take 8 g of the crude product of (6-amino-purin-9-yl)-ethyl acetate, add 200 ml of ethanol, heat to boiling, and filter while hot to remove alcohol-insoluble matter. Add an appropriate amount of activated carbon to the filtra...
Embodiment 2
[0136] Synthesis of Example 2 Compound II
[0137] (a) Synthesis of 3-(6-amino-purin-9-yl)-propionic acid ethyl ester
[0138]Adenine (10.8g, 0.08mol), absolute ethanol (280ml), and benzene (34ml) were placed in a three-necked flask, heated and distilled, and after collecting about 93ml fractions, metal sodium (80mg) was added. After no bubbles were generated, Slowly add 25.6ml of ethyl acrylate dropwise, after dropping, heat to reflux, monitor the reaction process by TLC, after 10 hours of reaction, distill and concentrate the reaction solution to precipitate a white solid, cool, filter with suction, recrystallize from ethanol, collect the product, weighing 17.7g, Yield 94.1%, melting point 164~166 ℃, literature [8] 167~168℃. 1 H-NMR (solvent CD 3 COOD): δ: 1.189~1.217 (t, J=7, 7, 2”-H), 4.121~4.163 (q, J=7, 7, 7, 1”-H) 3.070~3.095 (t, J= 6.5, 6, 3'-H), 4.636~4.661 (t, J=6.5, 6, 2'-H), 2.096~2.109 (solvent CD3COOD), 5.075 (solvent CD3COOD), 8.415 (s, 8-H) , 8.471 (s, 2-H...
preparation Embodiment 3
[0158] Formulation Example 3 Tablets are prepared according to methods known in the art, and each tablet contains the following ingredients:
[0159] Compound I 50mg
[0160] Lactose 60mg
[0162] Polyvinylpyrrolidone 120mg
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com