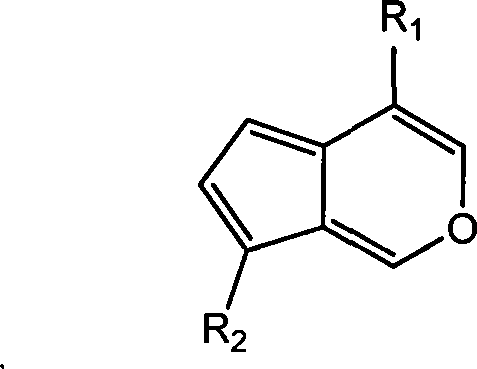
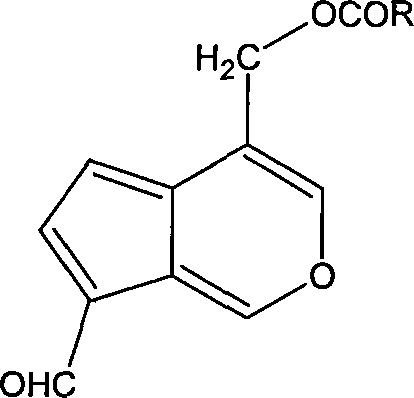
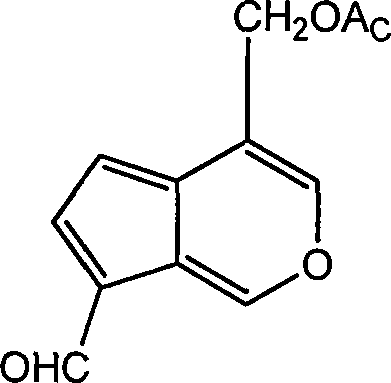
Application of iridoids compound in preparing medicine for treating benign prostate hyperplasia
A technology for iridoids and benign prostatic hyperplasia, which can be used in drug combinations, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of affecting clinical and market promotion, high price, and restricting the use of the elderly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Preparation and structure identification of the compounds of the present invention
[0068] 1. Extraction and separation
[0069] Coarse rhizome powder (6kg) was extracted by percolation with 95% ethanol as solvent, and 30L of percolation solution was collected and concentrated under reduced pressure to obtain 1500g of total extract. The total extract was dispersed in 500ml of water, extracted with petroleum ether (60~90℃), ethyl acetate and n-butanol in turn, and the solvent was recovered under reduced pressure to obtain 290g of petroleum ether (60~90℃) extract, which was extracted with ethyl acetate 130g of extract, 200g of n-butanol extract. (1) Further separation of petroleum ether extract
[0070] 100 g of the petroleum ether (60-90° C.) extract was subjected to silica gel column chromatography (gradient elution of petroleum ether→petroleum ether / ethyl acetate→ethyl acetate→acetone), and crudely divided into 204 parts.
[0071] PE51 # ~65 # After the...
Embodiment 2
[0119] Example 2 Preparation of an anti-benign prostatic hyperplasia capsule:
[0120] Take 1 kg of compound A and 6 kg of compound B, add an appropriate amount of starch, mix evenly, dry granulate, and divide into capsules after granulating.
[0121] The beneficial effects of the present invention are demonstrated below by the activity test.
experiment example 2
[0125] Experimental Example 2 Compound B of the present invention (PE116 # ) on the inhibition of 5α-reductase activity. The experimental method is the same as that of Experimental Example 1.
[0126] T
sample
T+h-DMEM
0.1mM
Cells
0.1mM
Compound B
100ug / ml
OD value
average
T(ng / ml)
2.184
2.077
2.130
5.014
2.156
2.169
2.162
4.695
2.187
2.196
2.192
4.421
2.056
1.970
2.013
6.383
[0127] DHT
sample
Cells
0.1mM
T
0.1mM
Compound B
100ug / ml
OD value
average
DHT(ng / ml)
1.603
1.656
1.629
1.009
1.029
1.098
1.064
5.405
1.207
1.200
1.203
3.572
[0128] The results show that the sample...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com