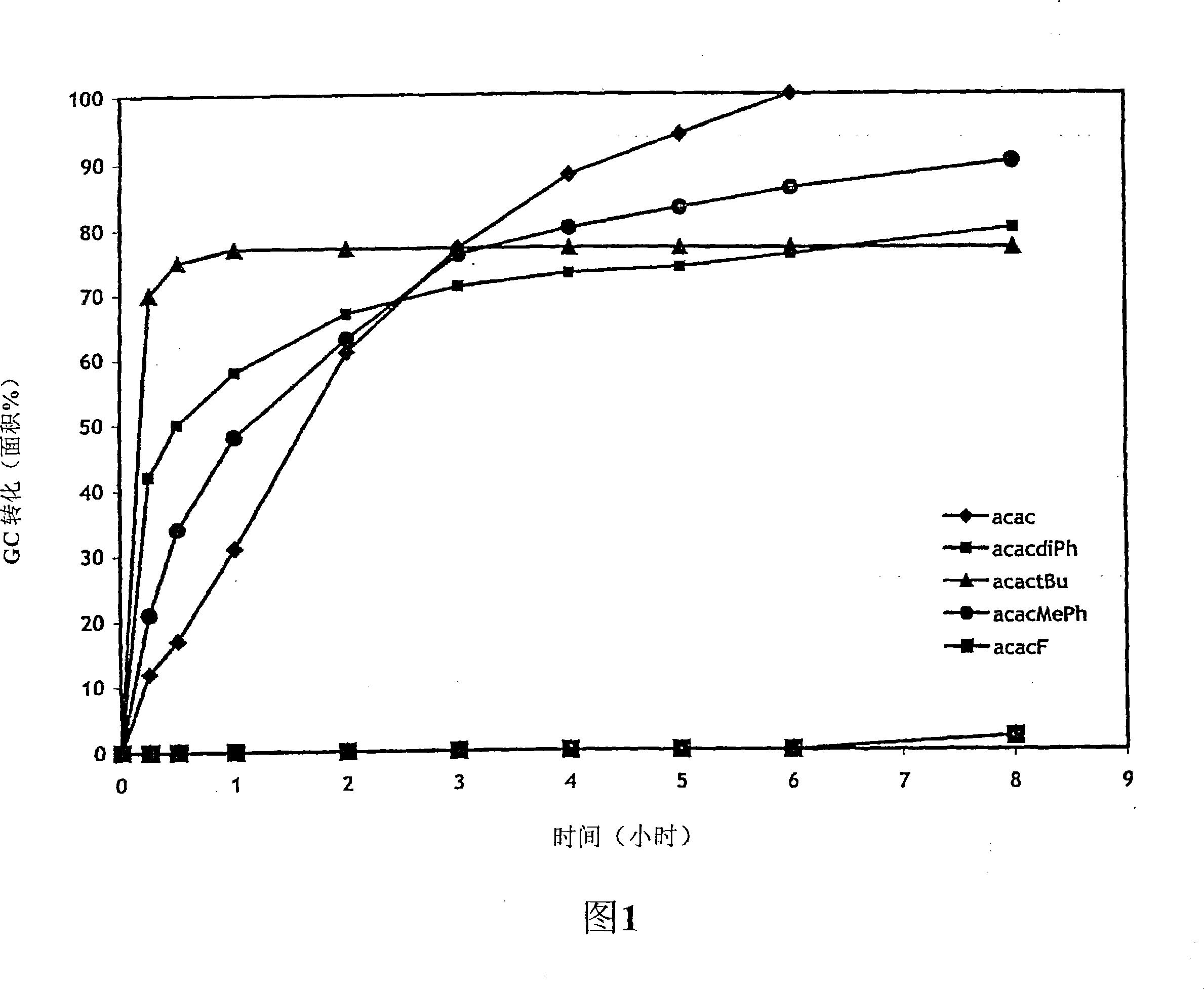
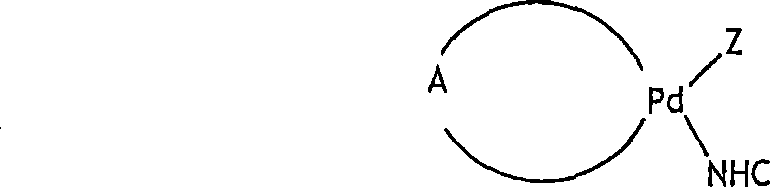
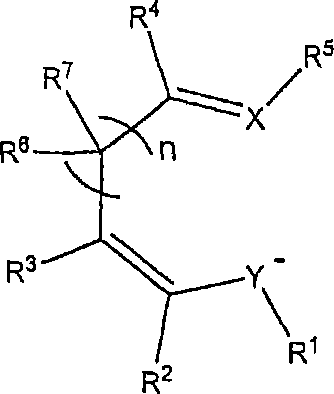
Nucleophilic heterocyclic carbene derivatives of pd(acac)2 for cross-coupling reactions
A technology of heterocyclic carbene and reaction, applied in the field of manufacturing these complexes, can solve the problems of laborious synthesis, difficulty, high cost, etc., and achieve the effect of low catalyst concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Example 1 Synthesis of (IPr)Pd(acac) 2 (Pd complex 1)
[0161] In the glove box, to a Schlenk flask equipped with a magnetic bar was added free carbene IPr (855 mg, 2.2 mmol), Pd(acac) 2 (609mg, 2mmol) and anhydrous toluene (30mL), sealed with a rubber cap. The mixture was stirred at room temperature for 2 hours. The solvent was evaporated in vacuo and THF (25 mL) was added. The solution was filtered and the solid was washed with THF (2 x 5 mL). The solvent was evaporated in vacuo; the complex was then triturated with cold pentane (25 mL), and the solution was filtered. Recrystallization from a chloroform / pentane mixture (25 / 75) gave 1.28 g (93%) of the title compound. 1 H-NMR (400MHz, C 6 D. 6 ): δ7.28-7.24(m, 2H), 7.18(d, J=8.0Hz, 4H), 6.47(s, 2H), 5.90(s, 1H), 4.78(s, 1H), 2.88(q, J=6.8 Hz, 4H), 2.63(d, J=0.8Hz, 3H), 2.01(d, J=0.8Hz, 3H), 1.63(s, 3H), 1.35(d, J=6.8, 12H), 1.31 (s, 3H), 0.97 (d, J=6.8, 12H). 13 C-NMR (100MHz, C 6 D. 6 ): 207.5, 192.9, 188....
Embodiment 2
[0162] Example 2 One-pot synthesis of (IPr)Pd(acac)Cl (Pd complex 2)
[0163] In the glove box, add free carbene IPr (2.73 g, 7 mmol), Pd(acac) to a Schlenk flask equipped with a magnetic bar 2 (1.53g, 5mmol) and dry dioxane (50mL), sealed with a rubber cap. The mixture was stirred at room temperature for 2 hours. Then, 1.25 mL of HCl in 4M dioxane was injected into the solution, and the mixture was stirred at room temperature for another 2 hours. The solvent was then evaporated in vacuo and diethyl ether was added until no more solid dissolved (20 mL). The solution was filtered and the solid was washed with ether (2 x 10 mL). The solvent was evaporated in vacuo and the resulting powder was kept under vacuum overnight to yield 2.85 g (90%) of the title product. 1 H-NMR (400MHz, CDCl 3 ): δ7.51(t, J=7.6Hz, 2H), 7.35(d, J=8Hz, 4H), 7.12(s, 2H), 5.12(s, 1H), 2.95(q, J=6.4Hz, 4H), 1.84(s, 3H), 1.82(s, 3H), 1.34(d, J=6.4Hz, 12H), 1.10(d, J=6.4Hz, 12H). 13 C-NMR (100MHz, CDCl...
Embodiment 3
[0164] Example 3 One-pot synthesis of (IPr)Pd(acac)Cl (Pd complex 2)
[0165] In the glove box, add imidazolium salt IPr HCl (2.96 g, 7 mmol), Pd(acac) to a Schlenk flask equipped with a magnetic bar 2 (1.53g, 5mmol) and anhydrous dioxane (100mL). The flask was taken out from the glove box, placed in an oil bath at 100°C, and stirred with a magnetic stirrer for 6 hours. The solution then appeared clear with no solids remaining. The solvent was evaporated in vacuo and diethyl ether was added until no more solids dissolved. The solution was filtered and the solid was washed with ether (2 x 10 mL). The solvent was evaporated in vacuo to afford 2.99 g (95%) of the title compound as a yellow powder.
[0166] 1 H NMR (δ, 400MHz, CDCl 3 ): 7.51(t, J=7.8Hz, 2H), 7.35(d, J=7.8Hz, 4H), 7.12(s, 2H), 5.12(s, 1H), 2.95(q, J=6.4Hz, 4H ), 1.84 (s, 3H), 1.82 (s, 3H), 1.34 (d, J=6.4Hz, 12H), 1.10 (d, J=6.4Hz, 12H).
[0167] 13 C NMR (δ, 100MHz, CDCl 3 ): 187.1, 184.1, 156.4, 147.0, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com