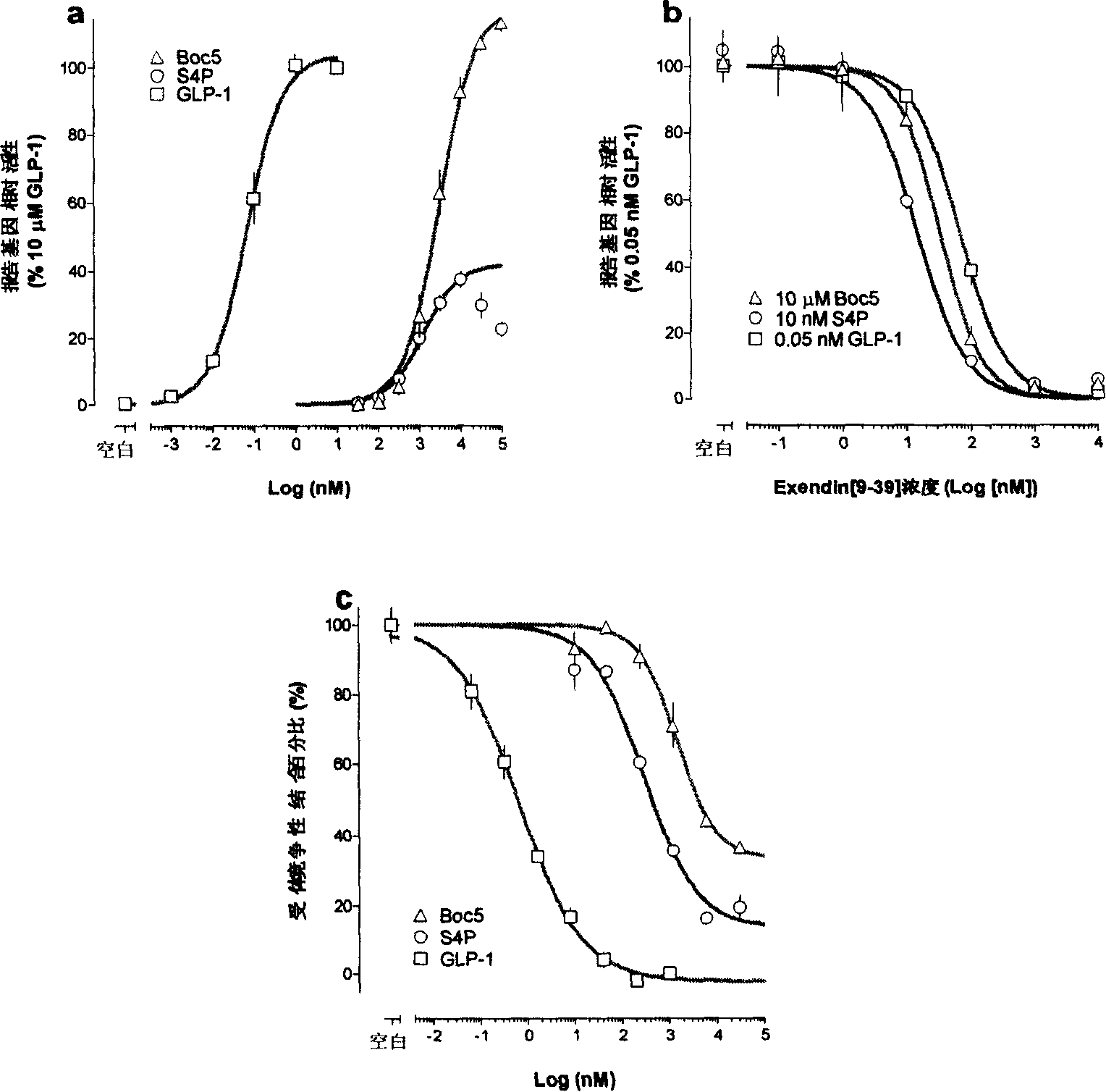
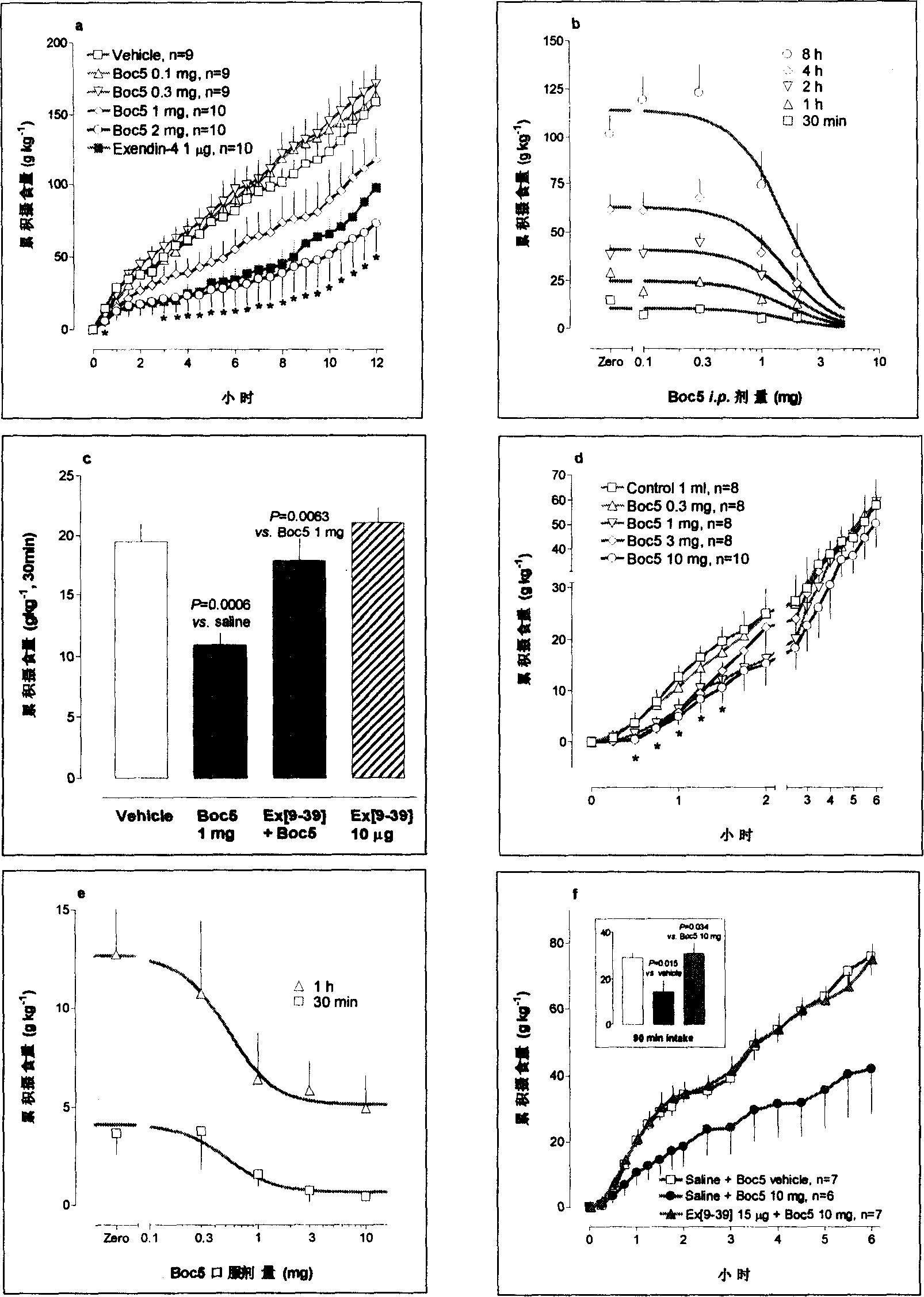
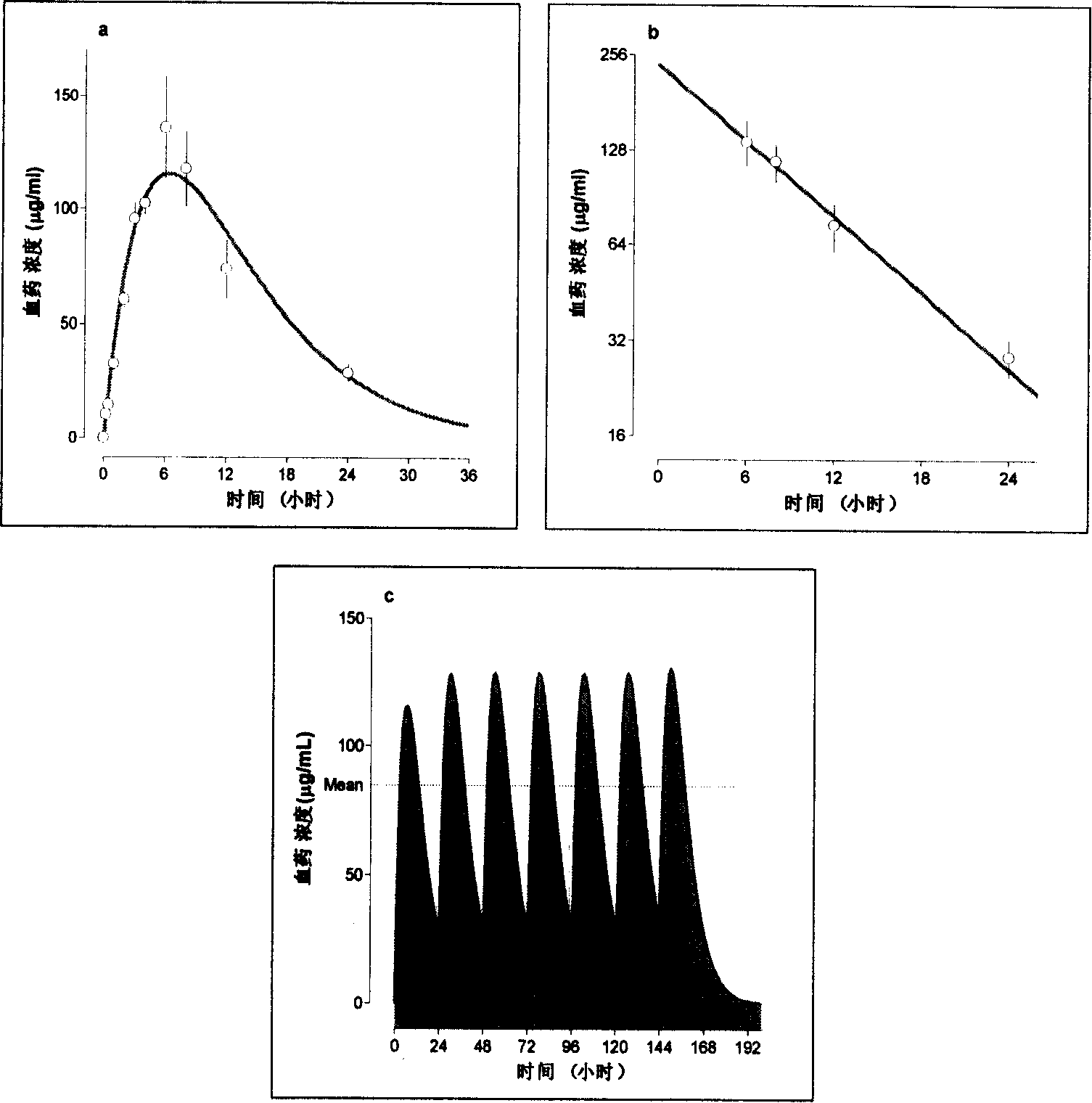
Compound with substituted tetratomic ring structure and medicine use thereof
A technology of four-membered rings and compounds, applied in the field of a class of compounds with substituted four-membered ring structures and their medical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] Embodiment 1: the preparation of compound S4P
[0167] NMR calibration: δH / C 7.26 / 77.0ppm (CDCl 3 ); δH / C 2.50 / 39.51ppm (DMSO-d 6 ).
[0168]
[0169] The compound Wang516 (1g) was dissolved in an appropriate amount of DMSO, placed under a 150W high-pressure mercury lamp for 3 days, added 1ml of water, and continued to irradiate for 7-10 days, during which the reaction was detected and tracked by HPLC. After the reaction was completed, the solvent was removed by lyophilization, and the residue was separated by column chromatography. Compound S4P was obtained as a light yellow powdery solid.
[0170] 1 HNMR (300MHz, DMSO-d 6 ) 10.053 (2H, br.s), 8.630 (2H, br.s), 8.090 (2H, dd, J 1 = 4.8Hz,J 2 = 1.2Hz), 8.029 (2H, dd, J 1 = 3.6Hz,J 2 =1.2Hz), 7.605(4H, d, J=8.4Hz), 7.395(4H, d, J=8.1Hz), 7.31(2H, m), 7.280(2H, br.s), 7.260(2H, m ), 7.206(2H, br.d, J=8.1Hz), 4.987(2H, br.s), 3.244(6H, s), 2.740(2H, m), 1.815(4H, m), 1.703(4H, m), 1.652 (4H, m), 1.526 (4H, m)...
Embodiment 2
[0172] Embodiment 2: the preparation of compound S4P and its isomer
[0173]
[0174] The compound Wang516 (10 g) was dissolved in an appropriate amount of DMSO, placed under natural light at room temperature for 30-90 days, lyophilized to remove the solvent, and the residue was separated by HPLC to obtain a small amount of light yellow powdery solid compound S4P. conformers and S4P.
[0175] 1 HNMR (300MHz, DMSO-d 6 ) 10.125 (2H, br.s), 8.025 (2H, d, J=4.8Hz), 7.921 (2H, d, J=2.9Hz), 7.667 (4H, br.s), 7.251 (2H, m), 7.220 (2H, br.s), 6.983 (2H, d, J=7.7Hz), 6.908 (2H, d, J=7.7Hz), 5.269 (2H, br.s), 3.335 (6H, s), 2.779 (2H, m), 1.832 (4H, m), 1.715 (4H, m), 1.677 (4H, m), 1.527 (4H, m).
[0176] 13 CNMR (75MHz, DMSO-d 6 ) 174.8, 174.7, 164.7, 159.5, 149.9, 142.1, 136.5, 135.0, 131.7, 129.3, 128.7, 127.6, 121.8, 120.1, 118.4, 112.0, 64.7, 55.1, 51.8, 45.3, 30.1, 25.7.
[0177] The NMR data of S4P are the same as Example 1.
Embodiment 3
[0178] Example 3: Preparation of Compound S4P Using a Catalyst
[0179]
[0180] Dissolve compound Wang516 (20 mg) in an appropriate amount of DMSO, add 25 mg of benzophenone, place it under a 150w high-pressure mercury lamp for 1 day, lyophilize to remove the solvent, and separate the residue by column separation to obtain light yellow powdery solid compound S4P .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com