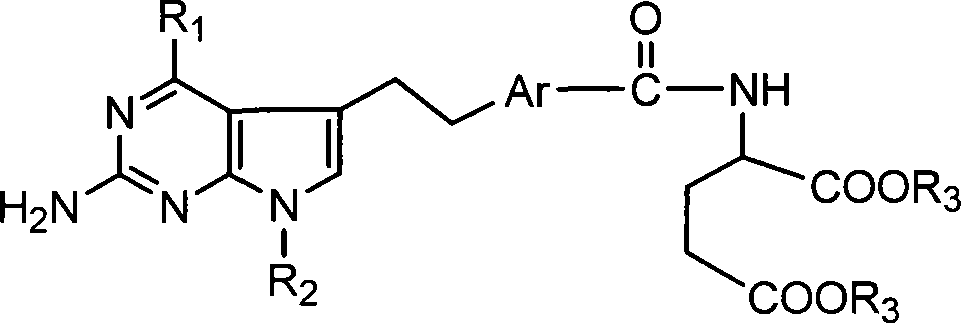
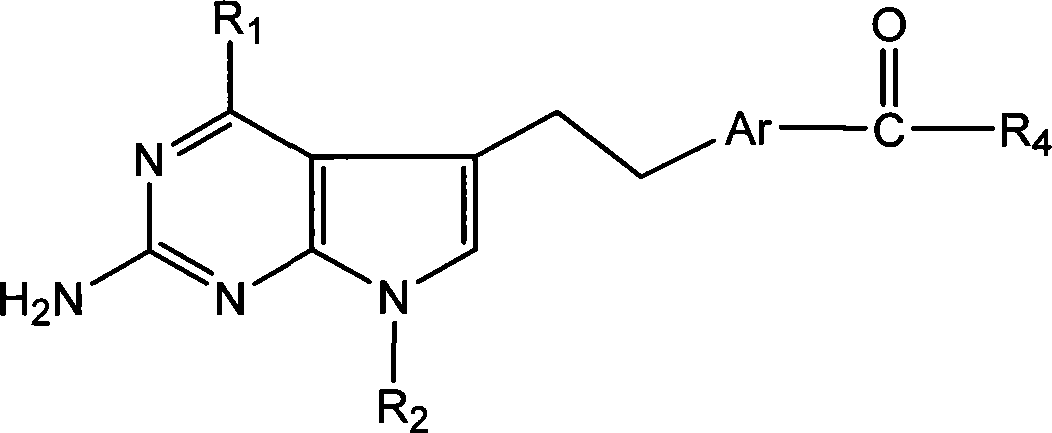
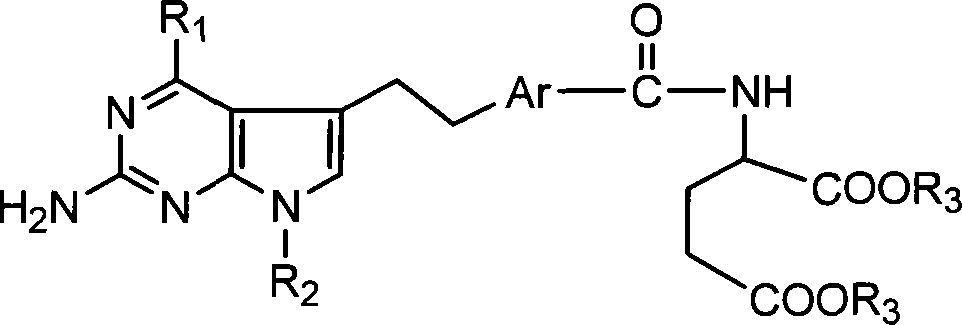
Antineoplastic medicament antifolate, its salt and midbody
An anti-folate agent and anti-tumor technology, which is applied in the field of anti-tumor drug anti-folate agent and its salts and intermediates, and compounds, can solve the problems of solid tumors with little effect, unsatisfactory treatment effect, and strong disease, and achieve Strong growth inhibitory activity, the effect of overcoming the problem of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The embodiments of the present invention are described in detail below: the present embodiment is implemented under the premise of the technical solution of the present invention, and detailed implementation and specific operation process are provided, but the protection scope of the present invention is not limited to the following implementation example.
[0031] This type of antifolate of the present invention and salt thereof can be synthesized by various methods, hereinafter referred to as R 1 =NH 2 and R 2 =CH 3 , or R 1 =NHCH 3 and R 2 =H, Ar is 1,4-phenyl or 2,5-thienyl as an example to illustrate the synthesis route of the present invention, but not limited to this functional group.
[0032] step 1
[0033] Synthesis of 4-[3-(2,4-diamino-6-methylamino-pyrimidin-5-yl)-4-nitro-butyl]-benzoic acid methyl ester 1
[0034] Reaction formula:
[0035]
[0036] 4-(4-Nitro-but-3-enyl)-benzoic acid methyl ester (0.69g, 2.93mmol) and 2,4-diamino-6-(methylamino...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com