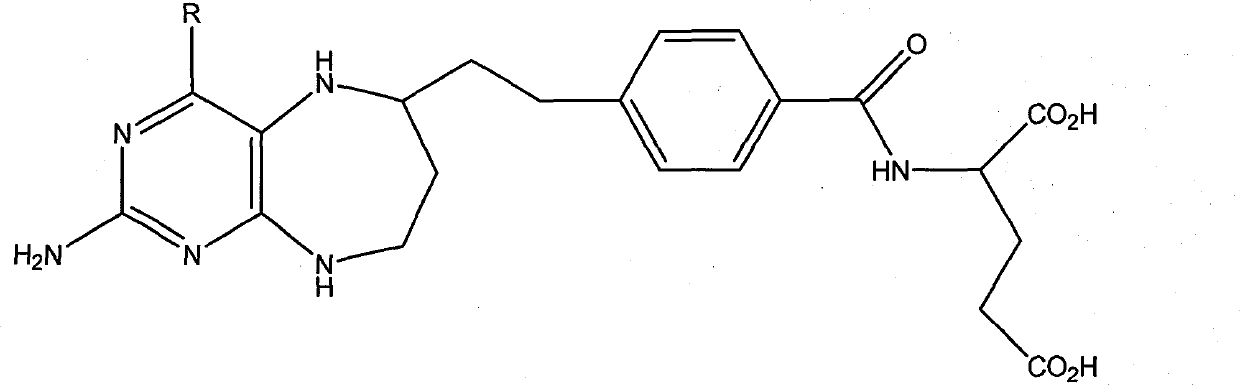
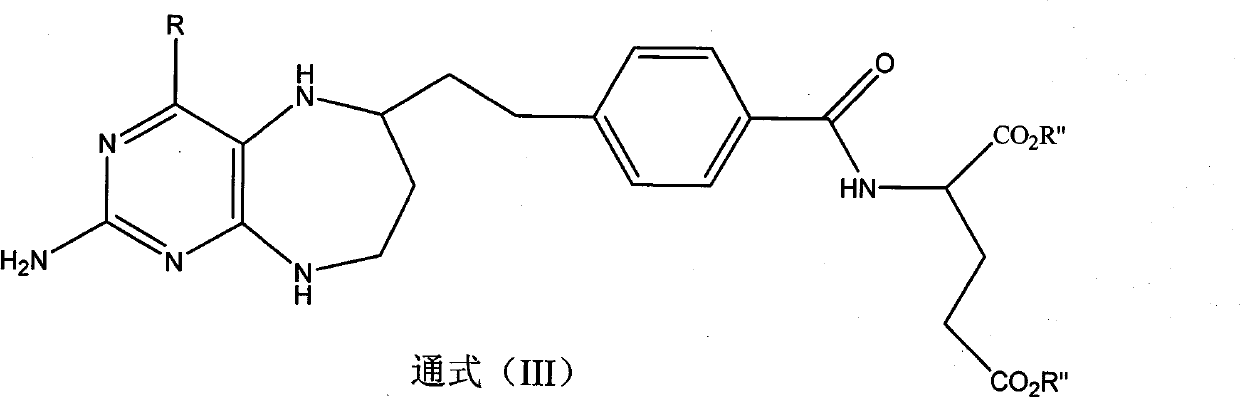
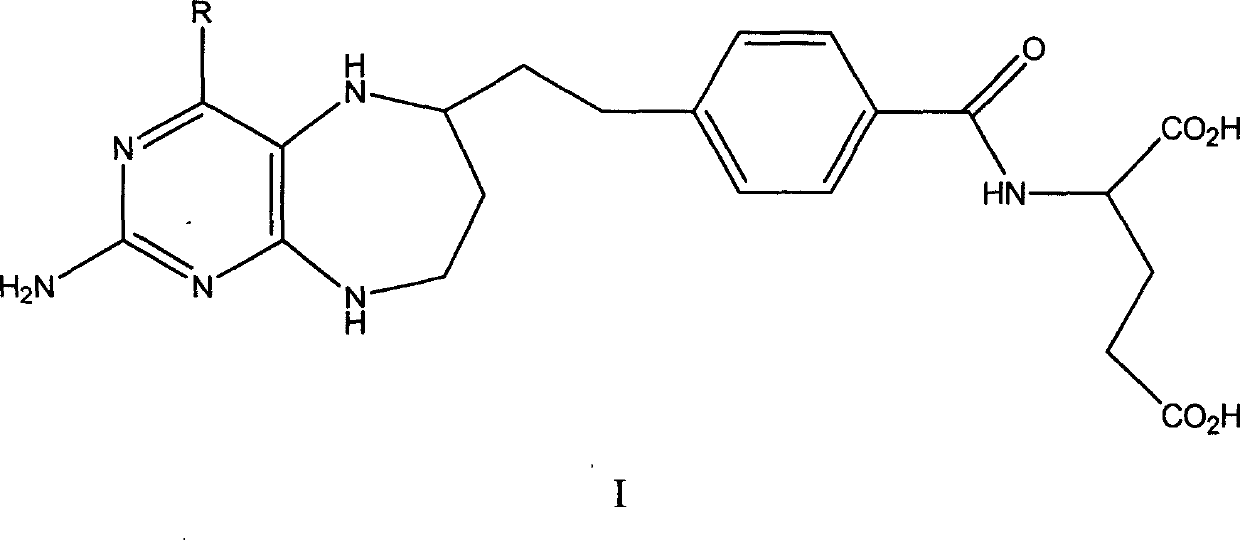
Folacin analogue, and salt of folacin analogue in use for medical treatment
An analogue, folic acid technology, applied in the field of chemistry, can solve the problems of solid tumors with little effect, strong, unable to obtain satisfactory therapeutic effect, etc., and achieve the effect of strong growth inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of 4-chloro-2-amino-6-(3-hydroxy-4-enyl-1-pentylamino)-pyrimidine (1)
[0041] 50mmol 4,6-dichloro-2-amino-pyrimidine and 5mmol~60mmol 3-hydroxyl-4-en-1-pentylamine were dissolved in 200ml ethanol and 51mmol triethylamine was added, N 2 Reflux under protection for 4h. Remove the solvent and add CH to the residual solid 2 Cl 2 The solvent was dissolved, and the insoluble matter was removed by filtration, the filtrate was concentrated, and the residue was subjected to silica gel column chromatography. EluentMeOH / CH 2 Cl 2 = 2 / 98. 8.31 g of the product was obtained with a yield of 73%.
[0042] 1 H NMR (CDCl 3)δ: 1.65~1.85(2H,dm), 3.30-3.63(m,2H), 4.2(1H,dm), 4.97(2H,sb), 5.03(1H,d), 5.27(1H,d), 5.80 (1H, s), 5.91 (1H, m).
Embodiment 2
[0044] Synthesis of 2-amino-4-methoxy-6-(3-hydroxy-4-enyl-1-pentylamino)-pyrimidine (2)
[0045] In 100ml methanol, add 6.48g (120mmol) sodium methylate and 5g (22mmol) 4-chloro-2-amino-6-(3-hydroxyl-4-enyl-1-pentylamino)-pyrimidine, N 2 Reflux for 48h under protection, remove most of the solvent under reduced pressure, add 100ml saturated NH 4 Cl aqueous solution with CH 2 Cl 2 Extraction (100mlx3), organic layer with anhydrous Na 2 SO 4 dry. Concentration gave 3.13 g of a light yellow solid, with a yield of 63.6%.
[0046] 1 H NMR (CDCl 3 )δ: 1.61-1.80 (2H, dm), 3.21-3.62 (2H, dm), 3.80 (3H, S), 4.19 (1H, d), 4.73 (2H, S, NH 2 ), 4.88 (1H, S, NH), 5.09 (1H, d), 5.25 (1H, d), 5.14 (1H, s), 5.88 (1H, m).
Embodiment 3
[0048] 4-[5-(2-Amino-4-methoxy-pyrimidine-6-amino)-3-oxo-pentyl]-benzoic acid methyl ester (3)
[0049] 560mg (2.5mmol) 2-amino-4-methoxy-6-(3-hydroxy-4-enyl-1-pentylamino)-pyrimidine, 720.5mg (2.75mmol) methyl 4-iodobenzoate, 577.5 mg(6.87mmol)NaHCO 3 , 28 mg (0.125 mmol) Pd (AcO) 2 , 885.5 mg (2.75 mmol) Bu 4 NBr and 0.5 g of crushed 4A molecular sieves, dissolved in 20 ml of anhydrous DMF, N 2 Stir at room temperature under protection for 72h, filter, and remove the solvent from the filtrate under reduced pressure to obtain a brownish-red solid, which is eluted by gradient elution of silica gel column chromatography 1.CH 2 Cl 2 , 2. CH 2 Cl 2 / MeOH (98:2) gave 0.77g of slightly light brown solid, yield 86%, mp110-113℃, R f =0.489(CH 2 Cl 2 / MeOH=20:1).
[0050] 1 H NMR (CDCl 3 )δ: 2.66(2H, t), 2.74(2H, t), 2.93(2H, t), 3.46(2H, m), 3.77(3H, s), 3.88(3H, s), 4.67(2H, s , NH 2 ), 4.95 (1H, s, NH), 5.09 (1H, s), 7.21 (2H, d), 7.92 (2H, d).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com