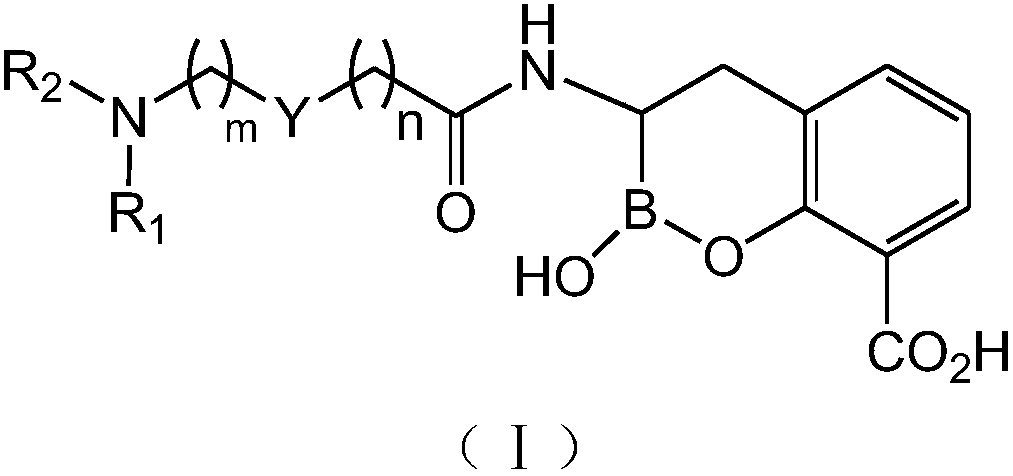
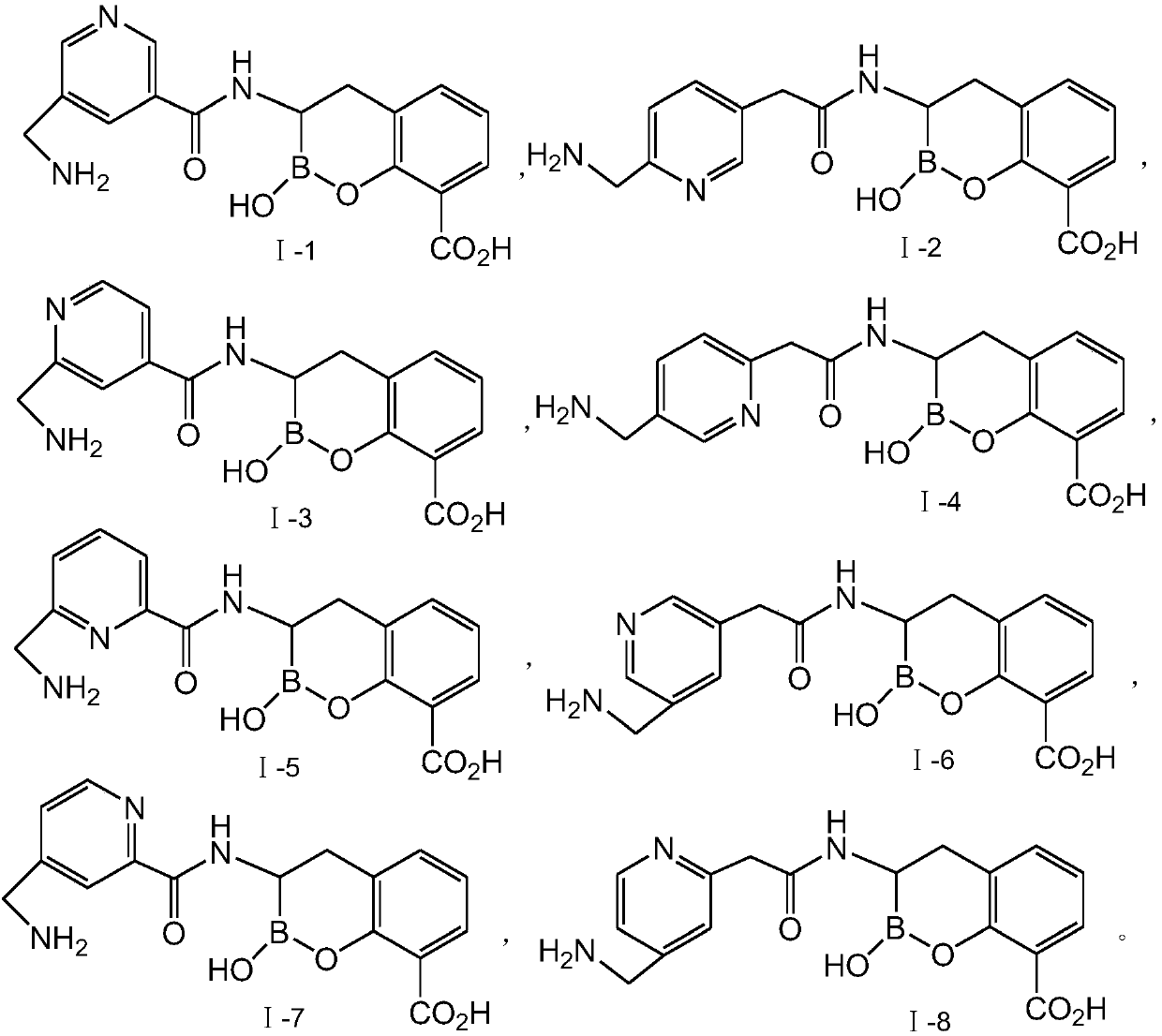
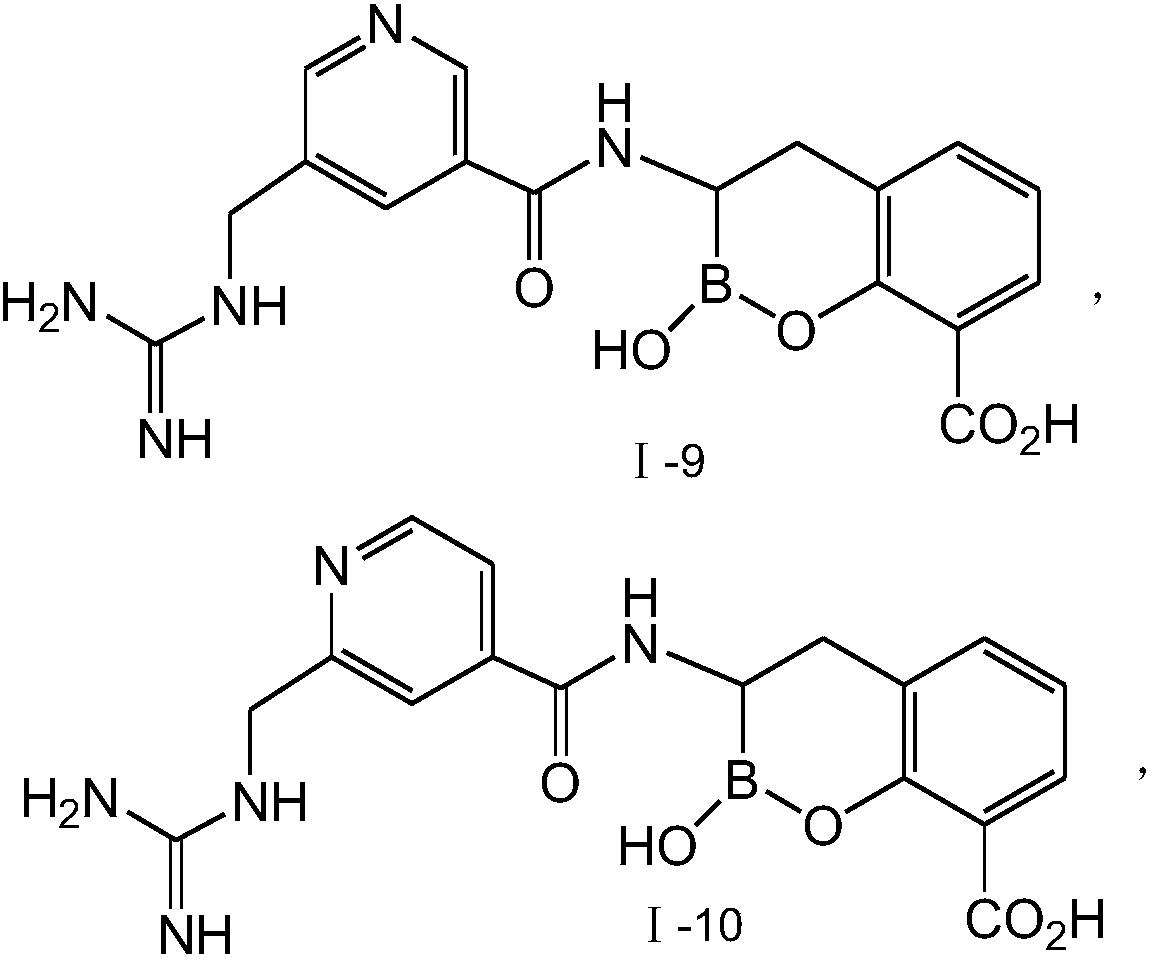
Novel broad-spectrum β-lactamase inhibitors
A lactamase and lactam technology, applied in the field of medicine, can solve the problems of insufficient inhibition of β-lactamase diversity, narrow enzyme inhibition activity, etc., and achieve great social and economic benefits, strong growth inhibition activity, and recovery of antibacterial properties. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of compound I-1
[0041]
[0042] Compound Ⅰ-1 is compound Ⅰ in which m=1, n=0, Y is 3,5-disubstituted pyridyl-C 5 h 3 N-, R 1 with R 2 The specific structure when both are hydrogen.
[0043] 1. Synthetic (2-tert-butoxycarbonyl)-5-aminomethylpyridine-3-carboxylic acid, i.e. compound A, synthetic route is as follows:
[0044]
[0045] Step 1. Synthesis of ethyl 5-cyanonicotinate (compound A-2) In a 300ml round bottom flask, pack ethyl 5-bromonicotinate (compound A-1, 4.60 g, 20.0 mmol), zinc cyanide (9.94 g, 84.6 mmol), tetrakis(triphenylphosphine)palladium(0) (4.69 g, 4.06 mmol) and DMF (100 mL). The mixture was heated at 90°C under argon for 15 hours. After cooling, the reaction was quenched with 10% ammonium acetate solution and extracted with ethyl acetate. The combined organic extracts were washed with water, brine and concentrated. The residue was purified by silica gel chromatography eluting with a gradient of 2 / 98 (V / V, v / v) ethyl acetate / ...
Embodiment 2
[0058] Synthesis of Compound I-2
[0059]
[0060] Compound Ⅰ-2 is compound Ⅰ in which m=1, n=1, Y is 2,5-disubstituted pyridyl-C 5 h 3 N-, R 1 with R 2 The specific structure when both are hydrogen.
[0061] , 1. Synthesis of 6-tert-butoxycarbonylaminomethyl-pyridin-3-yl-acetic acid, i.e. compound C, synthetic route is as follows:
[0062]
[0063] Step 1. Synthesis of (6-bromo-pyridin-3-yl)-ethyl acetate (Compound C-2) In a 500 mL round bottom flask, diisopropylamine (13.2 mL 93.92 mmol) was mixed with THF (41 mL) , and cooled to -78 degrees Celsius. And n-butyl lithium (2.5M in hexane; 38 mL, 91.20 mmol) was added, and the mixture was stirred for 30 minutes. Then 2-bromo-5-picoline (Compound C-1, 5 mL, 46.92 mmol) dissolved in 17 mL of THF was added. And the mixture was stirred for 2 hours. Diethyl carbonate (6.2 mL, 51.40 mmol) was then added, and the mixture was stirred overnight while gradually warming to room temperature. The reaction was quenched with sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com