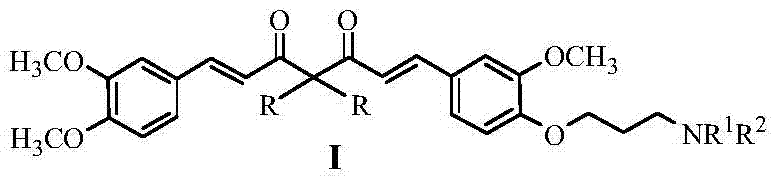
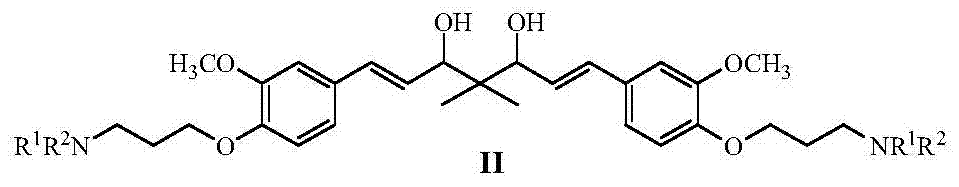
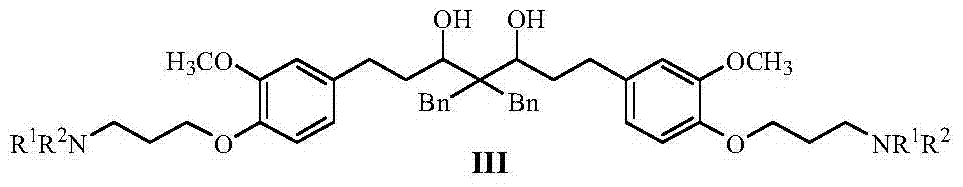
Curcumin analogue and its preparation and application
A technology of curcumin analogues and medicinal salts, which is applied in the application of antineoplastic drugs, intermediates for synthesizing the curcumin analogues and their preparation fields, can solve the problems of low water solubility, poor stability, rapid metabolism, etc. Achieve the effect of improving activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of 1-(3,4-dimethoxyphenyl)-3,5-diketone-1-hexene (A1)
[0044] Acetylacetone (16.3 mL, 160 mmol) and diboron trioxide (7.8 g, 112 mmol) were added to 100 mL of ethyl acetate, and the temperature was raised to 70° C. for 0.5 h. 3,4-Dimethoxybenzaldehyde (13.2g, 80mmol) and tri-n-butyl borate (21.6mL, 80mmol) were added slowly, and reacted at 70°C for 0.5h. Add 10 mL of ethyl acetate to 8 mL of n-butylamine, and dropwise complete. Reaction at 100°C for 1.5h. Cool down to 60°C, add 50mL of 0.4mol L -1 Hydrochloric acid, reaction 0.5h. After the reaction was completed, cool to room temperature, wash with water, extract the aqueous layer with ethyl acetate (30 mL×3), combine the organic phases, wash with saturated sodium chloride solution, and dry over anhydrous sodium sulfate. After filtration and concentration under reduced pressure, the obtained oil was separated and purified by column chromatography (eluent was petroleum ether-ethyl acetate). 6...
Embodiment 2
[0045] Example 2 1-(3,4-dimethoxyphenyl)-7-(3-methoxy-4-hydroxyphenyl)-1,6-heptadiene-3,5-dione (A2 ) preparation
[0046] 6-(3,4-dimethoxyphenyl)hex-5-ene-2,4-dione (10.0 g, 40.3 mmol) (A1) and diboron trioxide (4.8 g, 68.5 mmol) were added Into 160 mL of ethyl acetate, the temperature was raised to 70° C. for 0.5 h. Add 3-methoxy-4-hydroxybenzaldehyde (6.1g, 40.3mmol) and tri-n-butyl borate (18.4g, 79.0mmol) slowly, and react at 70°C for 0.5h. Piperidine (4mL, 40.5mmol) was added dropwise, and after the drop was completed, the temperature was raised to 100°C and reacted for 1.5h. Then cool down to 60°C, add 80mL of 0.4mol·L -1 Hydrochloric acid, reacted for 0.5h, after the reaction was completed, cooled to room temperature, washed with water, extracted the aqueous layer with ethyl acetate (30mL×3), combined the organic phases, washed with saturated sodium chloride solution, and dried over anhydrous sodium sulfate. After filtration and concentration under reduced pressure...
Embodiment 3
[0047] Example 3 1-(3,4-dimethoxyphenyl)-7-(3-methoxy-4-acetoxyphenyl)-1,6-heptadiene-3,5-dione Preparation of (A3)
[0048] 1-(3,4-dimethoxyphenyl)-7-(3-methoxy-4-hydroxyphenyl)-1,6-heptadiene-3,5-dione (A2) ( 5.0g, 13.1mmol), acetic anhydride (3.0mL, 32.8mmol) and pyridine (2.6mL, 32.8mmol) were added to 50mL of dichloromethane, and refluxed for 5h. After the reaction was completed, it was cooled to room temperature, adjusted to neutral pH with saturated aqueous sodium bicarbonate solution, and dried over anhydrous sodium sulfate. After filtration and concentration under reduced pressure, a yellow solid was obtained, which was recrystallized from ethyl acetate and petroleum ether to obtain 4.8 g of a yellow solid, with a yield of 86.4%. m.p.133-136℃, ESI-MS, m / z: 425.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com