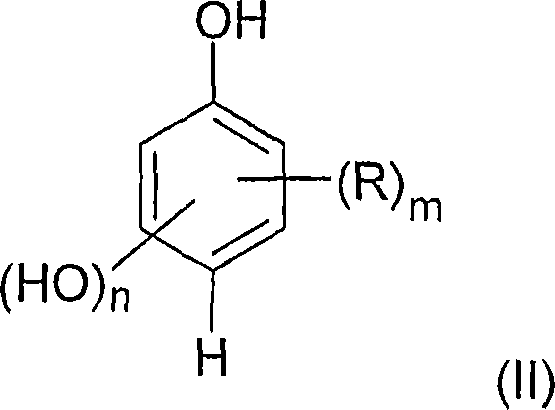
Method for the c-alkylation of hydroxyl aromatic compounds
A technology of aromatic compounds and hydroxylation, applied in the preparation of organic compounds, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve environmental pollution, alkylation and hydroxylation of aromatic compounds good selectivity difficulties etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
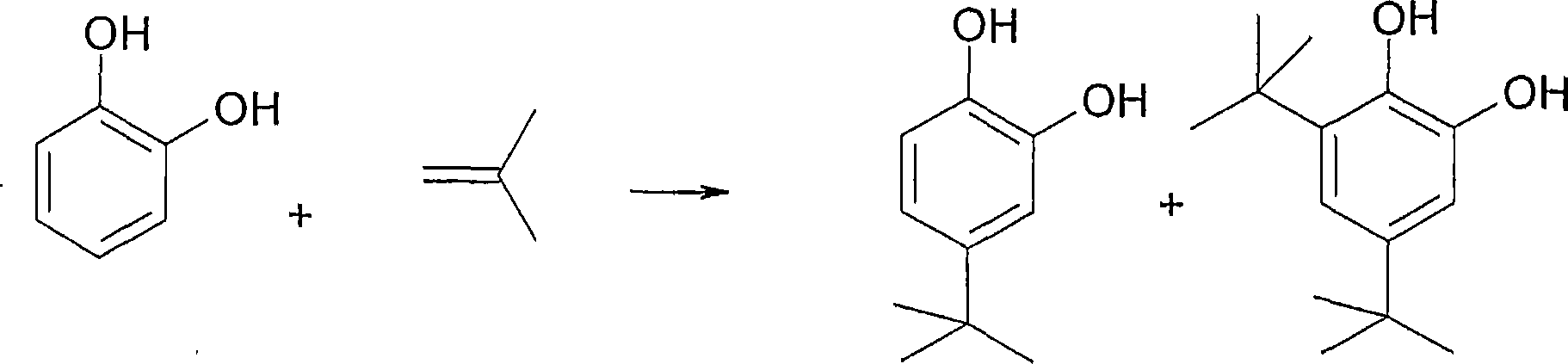
[0101] Synthesis of 4-(tert-butyl)catechol (TBC)
[0102] 50 g of catechol and a catalytic amount of acid were added sequentially to a 170 ml reactor. The reaction mixture was purged with nitrogen, heated to a temperature of 120° C., and then stirred. An isobutene pressure of 1 bar was then applied and the reaction was stopped when approximately 11 g of isobutene had been consumed. The reaction times necessary to consume these 11 grams are listed in Table (1A).
[0103] The reaction performed was as follows:
[0104]
[0105] The results obtained are listed in Table (1A):
[0106] Table (1A)
[0107] catalyst
[0108] The TOF of the catalyst was calculated (turnover frequency = moles of isobutylene converted per mole of catalyst per minute) and listed in Table (1B):
[0109] Table 1B
[0110] catalyst
[0111]From this it is evident that the catalyst of the invention, bis-trifluoromethanesulfonimide (TFSIH) acid, exhibits a particularly high ...
Embodiment 2
[0113] Selectivity of Phenol Alkylation Reaction
[0114] 10 grams of phenol and 6 grams of 2,2,4-trimethylpentene were added to a round bottom flask topped with a reflux condenser and equipped with a temperature sensor. The reaction mixture was heated to 38 °C followed by vigorous stirring. 8 mg of different catalysts were added to the reaction mixture. After one hour, the reaction was stopped, and the reaction product was analyzed.
[0115] The reaction performed was as follows:
[0116]
[0117] The results are listed in Table (2):
[0118] Table 2)
[0119] catalyst
DC 2,2,4-三甲基戊烯 (%)
Pair / o(4) / (3) selectivity
PTSA
45.5
1.27
TA
100
6.52
Trifluoroacetic acid (TFA)
18.2
-
Fuming sulfuric acid: H 2 SO 4 +20% SO 3
41.9
1.45
TFSIH
100
15.41
Methanesulfonic acid (MSA)
38
1.12
h 2 SO 4
47.2
1.37
[012...
Embodiment 3
[0123] Selectivity of Phenol Alkylation Reaction
[0124] The comparative results of the alkylation of phenol with 2,4,4-trimethylpentene (addition time: 60 minutes) catalyzed by 5 different acids (1500 ppm by weight) at 60° C. are listed in the table below:
[0125] table 3
[0126] catalyst
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com