Macromolecular bactericide and method for preparation
A technology of bactericide and polymer, applied in the field of polymer bactericide and its preparation, can solve rare problems and achieve the effect of good killing microorganism effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment is the preparation of chlorobutyryl chloride.
[0042] Add 40mL of thionyl chloride and 3g of anhydrous zinc chloride into a 150mL 3-neck round bottom flask equipped with mechanical stirring, start stirring, and quickly add 38mL of γ-butyrolactone to raise the temperature of the solution to 45°C. The reaction mixture was heated to 55°C and the reaction was stirred for 22 hours. The supernatant was transferred to a 150 mL round-bottomed flask for rotary vacuum evaporation and separation. The unreacted raw materials were evaporated at a residual pressure of 3 mmHg and a temperature of 80° C., and the obtained liquid was chlorobutyryl chloride.
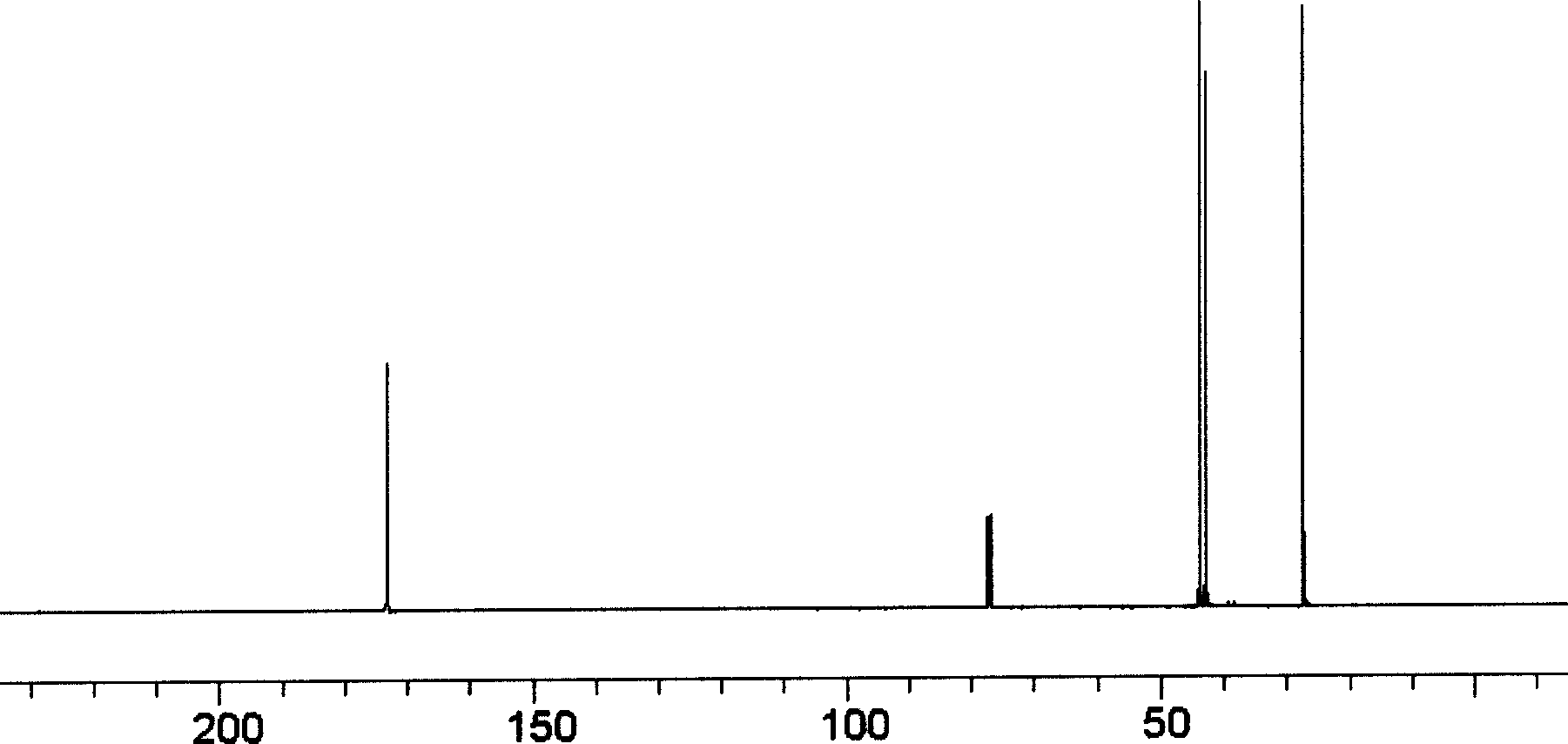
[0043] 13 CNMR ( figure 1 ) characterization only appears the following characteristic peaks: δ173.20 (ClCOCH 2 CH 2 CH 2 Cl), δ46.43(ClCOCH 2 CH 2 CH 2 Cl), δ27.8(ClCOCH 2 CH 2 CH 2 Cl), δ44.53(ClCOCH 2 CH 2 CH 2 Cl), indicating that chlorobutyryl chloride has been obtained.
Embodiment 2
[0045] This embodiment is the preparation of the intermediate product HP2Cl.
[0046] Add 100mL tetrahydrofuran and 20.0g BoltornH20 into a 250mL 3-neck round bottom flask equipped with mechanical stirring, start stirring, and add 29.9mL pyridine after the solid is dissolved. Slowly add 41.5mL of chlorobutyryl chloride dropwise in an ice-water bath, and react at room temperature for 24 hours after the dropwise addition, pour the reaction mixture into 1L of deionized water, a brown viscous solid precipitates, and then dissolves the precipitate in acetone , poured into 1L deionized water, and repeated 3 times. The obtained brown viscous solid was vacuum-dried at a residual pressure of 3 mmHg and a temperature of 80°C. 29.5 g of product were obtained (75.0% yield). Through element analysis, the product contains Cl 16.60% (weight).
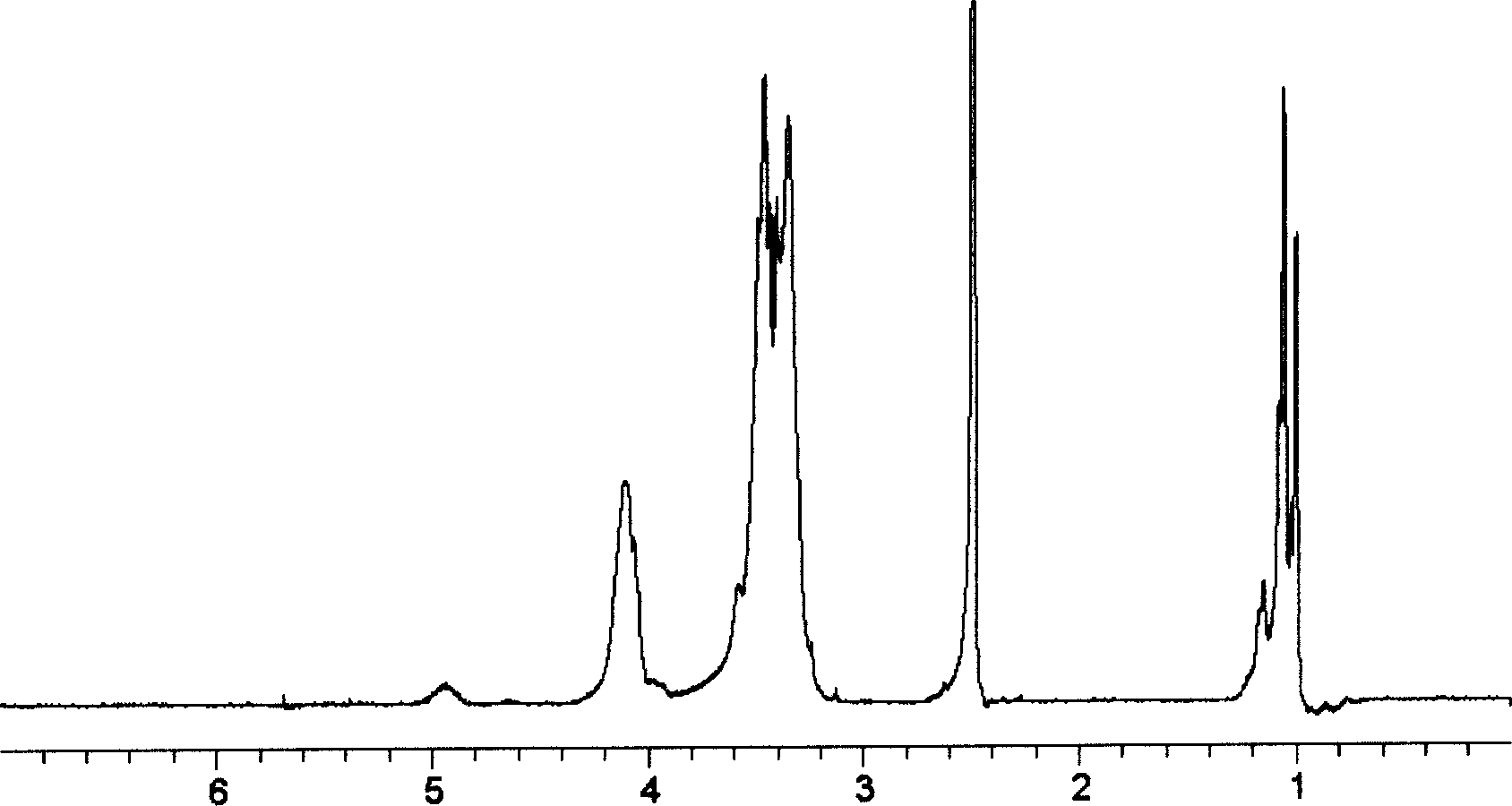
[0047] From 1 H NMR analysis shows that raw material Boltorn H 20 ( figure 2 ) of two hydroxyl characteristic peaks, δ4.94 (CH 2 OH) and δ3.46...
Embodiment 3
[0049] This embodiment is the preparation of the final product HP2N12.
[0050] Add 30.0mL dimethyl sulfoxide and 5.0gHP2Cl into a 150mL 3-neck round bottom flask equipped with mechanical stirring, start stirring, and add 12.6mL dodecyldimethyl tertiary amine (Jiangsu Feixiang Chemical Co., Ltd. product). Reacted at 80°C for 48 hours, cooled to room temperature, poured the reaction mixture into 300mL acetone, a light brown solid precipitated, then dissolved the precipitate in ethanol, poured into 300mL acetone, repeated 3 times. The obtained brown solid was vacuum-dried at a residual pressure of 3 mmHg and a temperature of 80° C. to obtain 6.3 g of the product (yield 63.0% by weight). Through elemental analysis, the product contains 3.22% (weight) of N.
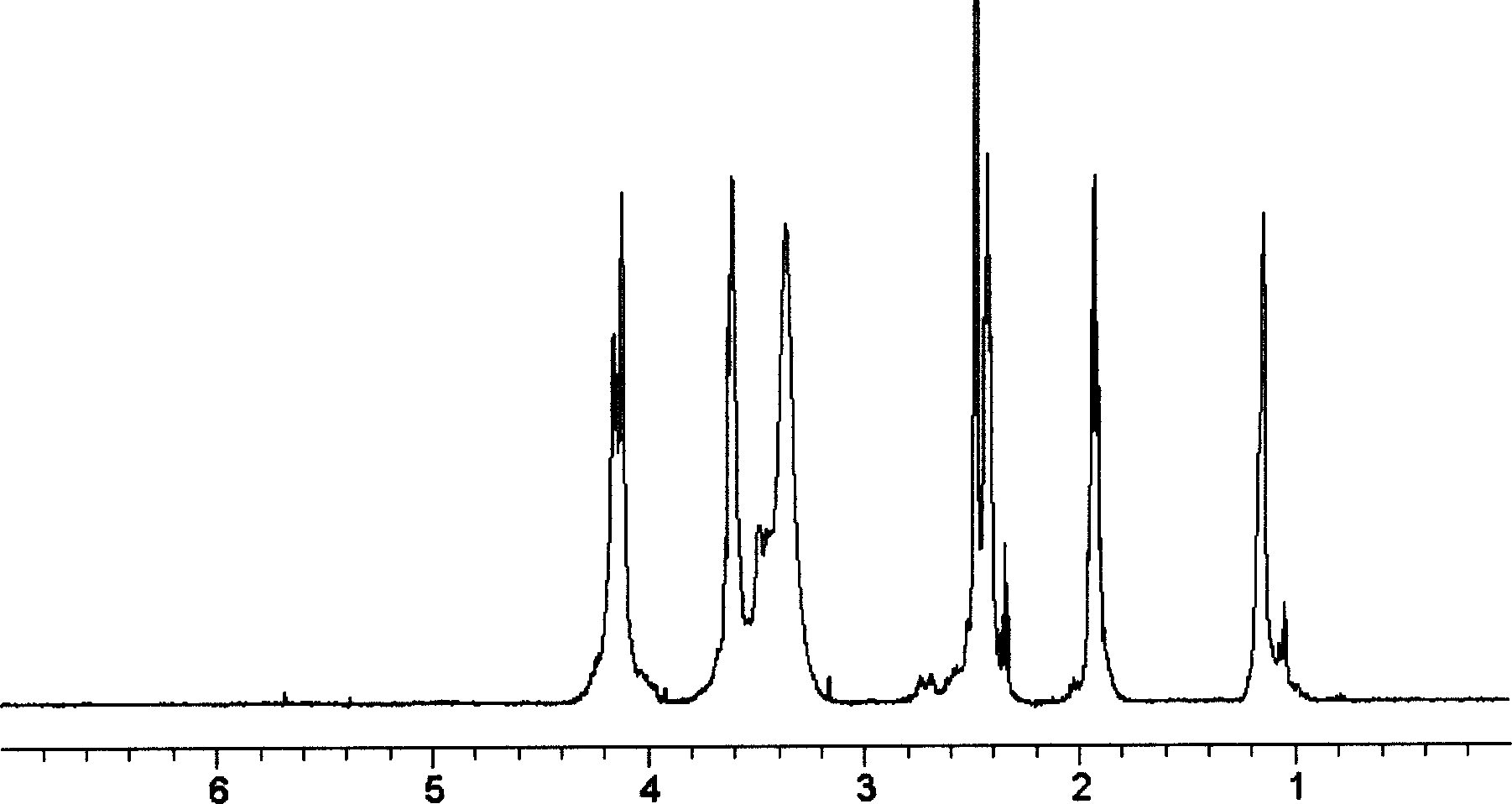
[0051] From 1 H NMR analysis shows that after the quaternization reaction (product HP N , Figure 4 ), HP2Cl ( image 3 ) The characteristic peak of δ3.64 disappears, and δ3.05 (CH 2 NCH 2 C 10 h 20 CH 3 ), δ1.23 (N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com